当前位置:
X-MOL 学术
›
Org. Biomol. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Asymmetric total syntheses of naturally occurring α,β-enone-containing RALs, L-783290 and L-783277 through intramolecular base-mediated macrolactonization reaction
Organic & Biomolecular Chemistry ( IF 3.2 ) Pub Date : 2020/03/09 , DOI: 10.1039/d0ob00237b Joy Chakraborty 1, 2, 3, 4 , Ankan Ghosh 1, 1, 2, 3, 4 , Samik Nanda 1, 2, 3, 4
Organic & Biomolecular Chemistry ( IF 3.2 ) Pub Date : 2020/03/09 , DOI: 10.1039/d0ob00237b Joy Chakraborty 1, 2, 3, 4 , Ankan Ghosh 1, 1, 2, 3, 4 , Samik Nanda 1, 2, 3, 4
Affiliation
|
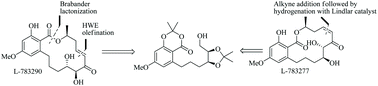
Asymmetric total synthesis of two naturally occurring α,β-enone containing RALs, L-783290 and L-783277 is described in this article. An E-selective Horner–Wadsworth–Emmons (HWE) olefination was used as a key reaction to construct the C7′–C8′ olefinic unsaturation in L-783290. An enantiopure alkyne addition to the aldehyde followed by Z-selective partial reduction was employed to construct the C7′–C8′ olefinic unsaturation in L-783277. Biomimetic lactonization reaction was used to construct the macrolactone core in both the target molecules.
中文翻译:

天然存在的含α,β-烯酮的RAL,L-783290和L-783277的不对称总合成,通过分子内碱基介导的大内酯化反应
本文描述了两种天然存在的含α,β-烯酮的RALs L-783290和L-783277的不对称全合成。一个Ë -选择性-沃兹沃思-埃蒙斯霍纳(HWE)烯用作键反应来构建Ç 7' -C 8'的烯属不饱和度在L-783290。的对映体纯炔除醛随后Ž -选择性部分还原是用于构造与c 7' -C 8'的烯属不饱和度在L-783277。仿生内酯化反应用于在两个靶分子中构建大内酯核。
更新日期:2020-03-26
中文翻译:

天然存在的含α,β-烯酮的RAL,L-783290和L-783277的不对称总合成,通过分子内碱基介导的大内酯化反应
本文描述了两种天然存在的含α,β-烯酮的RALs L-783290和L-783277的不对称全合成。一个Ë -选择性-沃兹沃思-埃蒙斯霍纳(HWE)烯用作键反应来构建Ç 7' -C 8'的烯属不饱和度在L-783290。的对映体纯炔除醛随后Ž -选择性部分还原是用于构造与c 7' -C 8'的烯属不饱和度在L-783277。仿生内酯化反应用于在两个靶分子中构建大内酯核。

























 京公网安备 11010802027423号
京公网安备 11010802027423号