当前位置:
X-MOL 学术
›
J. Mater. Chem. A
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Engineering the hydrogen evolution reaction of transition metals: effect of Li ions
Journal of Materials Chemistry A ( IF 11.9 ) Pub Date : 2020-03-06 , DOI: 10.1039/c9ta12926j Anku Guha 1, 2, 3 , Nisheal M. Kaley 1, 2, 3 , Jagannath Mondal 1, 2, 3 , Tharangattu N. Narayanan 1, 2, 3
Journal of Materials Chemistry A ( IF 11.9 ) Pub Date : 2020-03-06 , DOI: 10.1039/c9ta12926j Anku Guha 1, 2, 3 , Nisheal M. Kaley 1, 2, 3 , Jagannath Mondal 1, 2, 3 , Tharangattu N. Narayanan 1, 2, 3
Affiliation
|
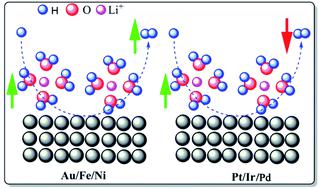
Though the role of supporting ions such as Li ions (Li+) in the electrochemical hydrogen evolution reaction (HER) has been extensively studied in the recent past, the fundamental mechanism influencing the HER kinetics of different metals under varied electrolyte conditions is still not known. Here we unveil the mechanism leading to a tunable HER on different metals under varying pH conditions, and it is established that despite the nominal role of counter anions, Li+ alone can tune both the HER thermodynamics and kinetics of metals. Different metals, namely Pt, Ir, Pd, Au, Fe, and Ni, are studied here to ensure the representation from both the sides of the Sabatier HER plot, under different pH conditions towards their HER efficacy at varying Li+ concentrations. Pt, Pd, and Ir showed suppression in their HER properties with increasing Li+ concentration, while the reverse phenomenon was observed on the rest of the metals. The metal–hydrogen (M–H) binding energy variation with Li+ is theoretically proven, and the Pt–H and Pd–H binding energy variations with Li+ containing electrolytes are experimentally demonstrated. Studies show that the tunability of the HER properties of both noble and non-noble metals can be achieved irrespective of the pH (0 and 13) and counter ions (TFSI−, Cl−, ClO4−, NO3− and OH−) by tuning the M–H bond energy using Li+, where this will be helpful in designing better electro-catalysts not only for the HER but also for other important reactions such as carbon dioxide reduction, nitrogen reduction, etc., where the HER is their competitor in aqueous medium.
中文翻译:

工程化过渡金属的氢释放反应:锂离子的作用
虽然支撑离子如Li离子(Li作用+在电化学析氢反应)(HER)已被广泛地在最近的过去的研究,多样电解质的条件下影响HER不同金属的动力学的基本机制仍然未知。在这里,我们揭示了在不同pH条件下在不同金属上产生可调谐HER的机理,并且已确定,尽管抗衡阴离子具有标称作用,但仅Li +可以同时调节金属的HER热力学和动力学。本文研究了不同的金属,即Pt,Ir,Pd,Au,Fe和Ni,以确保在不同的pH条件下,从Sabatier HER图的两侧代表其在Li +变化时的HER功效。浓度。随着Li +浓度的增加,Pt,Pd和Ir的HER性能受到抑制,而其余金属则观察到相反的现象。从理论上证明了金属与氢(MH)结合Li +的结合能变化,并通过实验证明了Pt-H和Pd-H与含Li +电解质的结合能变化。研究表明,可不论达到的pH值(0至13)和抗衡离子的HER既贵金属和非贵金属的特性的可调性(TFSI - ,氯-,CLO 4 -,NO 3 -和OH - )通过使用Li调节MH键的能量+,这将有助于设计更好的电子催化剂,不仅适用于HER,而且还适用于其他重要反应,例如二氧化碳还原,氮还原等,其中HER是其在水性介质中的竞争对手。
更新日期:2020-03-06
中文翻译:

工程化过渡金属的氢释放反应:锂离子的作用
虽然支撑离子如Li离子(Li作用+在电化学析氢反应)(HER)已被广泛地在最近的过去的研究,多样电解质的条件下影响HER不同金属的动力学的基本机制仍然未知。在这里,我们揭示了在不同pH条件下在不同金属上产生可调谐HER的机理,并且已确定,尽管抗衡阴离子具有标称作用,但仅Li +可以同时调节金属的HER热力学和动力学。本文研究了不同的金属,即Pt,Ir,Pd,Au,Fe和Ni,以确保在不同的pH条件下,从Sabatier HER图的两侧代表其在Li +变化时的HER功效。浓度。随着Li +浓度的增加,Pt,Pd和Ir的HER性能受到抑制,而其余金属则观察到相反的现象。从理论上证明了金属与氢(MH)结合Li +的结合能变化,并通过实验证明了Pt-H和Pd-H与含Li +电解质的结合能变化。研究表明,可不论达到的pH值(0至13)和抗衡离子的HER既贵金属和非贵金属的特性的可调性(TFSI - ,氯-,CLO 4 -,NO 3 -和OH - )通过使用Li调节MH键的能量+,这将有助于设计更好的电子催化剂,不仅适用于HER,而且还适用于其他重要反应,例如二氧化碳还原,氮还原等,其中HER是其在水性介质中的竞争对手。



























 京公网安备 11010802027423号
京公网安备 11010802027423号