Journal of Organometallic Chemistry ( IF 2.3 ) Pub Date : 2020-03-09 , DOI: 10.1016/j.jorganchem.2020.121221 Nishamol Kuriakose , Sai V.C. Vummaleti , Alexander Genest , Notker Rösch
|
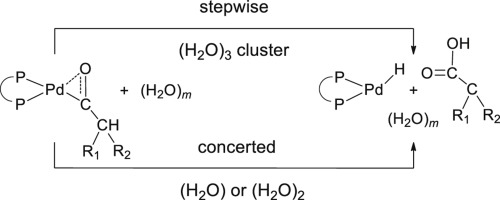
We explored computationally variations in the hydrolysis step of the Pd(II)-catalyzed hydroxycarbonylation of pentenoic acids for several ligands L2 = 1,2-bis[(di-R)phosphinomethyl]benzene where R = tert-butyl, methyl, or phenyl, referring to these ligands by DTBPX, DMPX, and DPPX, respectively. Thus far, most computational models invoked three H2O molecules in the crucial step which can occur in either a concerted or a stepwise fashion. We used density functional calculations to study systematically the effect of the number of water molecules (H2O)m, m = 1–3, on the nature of the crucial step, concerted vs stepwise. Accordingly, the concerted mechanism is preferred over the stepwise mechanism for m = 1. For m = 2, the stepwise mechanism is preferred for the ligand DTBPX, and the concerted mechanism for the ligands DMPX or DPPX. For m = 3, the stepwise mechanism is preferred for all three ligands under study and, importantly, also results in the overall lowest hydrolysis barriers. An energy decomposition analysis of these hydrolysis barriers, evaluating corrections due to larger basis set, dispersion interaction, and solvation, indicates that the latter term indeed is responsible for rendering adipic acid the most likely product.
中文翻译:

在Pd(II)催化的戊烯酸羟羰基化中模拟配体和溶剂化对水解变体的影响
我们探讨了几种配体L 2 = 1,2-双[(二-R)膦甲基]苯的Pd(II)催化戊烯酸羟羰基化水解步骤的计算差异,其中R = 叔丁基,甲基或苯基,分别指DTBPX,DMPX和DPPX的这些配体。迄今为止,大多数计算模型都在关键步骤中调用了三个H 2 O分子,这些分子可以以协同或逐步的方式发生。我们使用密度泛函计算系统地研究的水分子的数量的效果(H 2 O)米,米= 1-3,在关键步骤的性质,一致VS逐步地。因此,对于m = 1,优选协调机制优于逐步机制。对于m = 2,对于配体DTBPX优选逐步机制,对于配体DMPX或DPPX优选协同机制。对于m = 3,逐步机理对于所研究的所有三个配体都是优选的,并且重要的是,这也导致总体上最低的水解障碍。对这些水解壁垒的能量分解分析,评估了由于较大的基团,分散体相互作用和溶剂化而引起的校正,表明后一项确实有助于使己二酸成为最可能的产物。



























 京公网安备 11010802027423号
京公网安备 11010802027423号