Chemical Engineering Research and Design ( IF 3.9 ) Pub Date : 2020-03-07 , DOI: 10.1016/j.cherd.2020.03.001 Diana C. Vargas , Sebastián Salazar , José R. Mora , Kevin M. Van Geem , Daniela Almeida Streitwieser
|
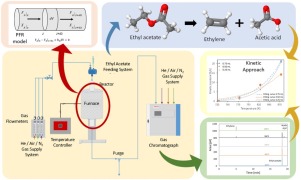
The thermal decomposition of ethyl acetate was experimentally studied in a newly designed fast pyrolysis set-up. The results were compared to theoretical calculations and literature values in order to proof the experimental concept. The reaction was carried out in a free fall tubular reactor with a residence time of 0.15 s. The identification and quantification of products stream composition was performed online using a GC-TCD/FID. First, an overview of the reaction rate at feed volumes of 0.25, 0.50 and 0.75 mL was obtained at reaction temperature between 400 to 600 °C in intervals of 50 °C. As a result mass transfer limitation for feeds larger than 0.5 mL were identified. For the second approach, a constant feed volume of 0.25 mL and temperatures between 420 to 550 °C were investigated. Using the experimental results, a global kinetic model is proposed for the thermal decomposition of ethyl acetate into ethylene and acetic acid through a first order unimolecular reaction. Also, theoretical calculations were performed at ωB97XD/6-311++G(d,p) level. A concerted mechanism through a six-membered transition state was identified in the reaction path. The theoretical and experimental activation energy values lie within the literature values between 193 and 213 kJ/mol.
中文翻译:

快速热解过程中乙酸乙酯热分解的实验和理论研究
在新设计的快速热解装置中,对乙酸乙酯的热分解进行了实验研究。将结果与理论计算和文献值进行比较,以证明实验概念。该反应在自由下落管式反应器中进行,停留时间为0.15秒。使用GC-TCD / FID在线进行产物流组成的鉴定和定量。首先,在400至600°C之间的反应温度(以50°C为间隔)下获得进料量为0.25、0.50和0.75 mL时的反应速率概览。结果,确定了大于0.5 mL进料的传质限制。对于第二种方法,研究了0.25 mL的恒定进料量和420至550°C的温度。利用实验结果,提出了通过一级单分子反应将乙酸乙酯热分解为乙烯和乙酸的整体动力学模型。同样,在ωB97XD/ 6-311 ++ G(d,p)水平上进行了理论计算。在反应路径中确定了通过六元过渡态的协调机制。理论和实验活化能值在193至213 kJ / mol的文献值范围内。


























 京公网安备 11010802027423号
京公网安备 11010802027423号