Tetrahedron ( IF 2.1 ) Pub Date : 2020-03-08 , DOI: 10.1016/j.tet.2020.131112 Rodolfo G. Fiorot , José Walkimar de M. Carneiro
|
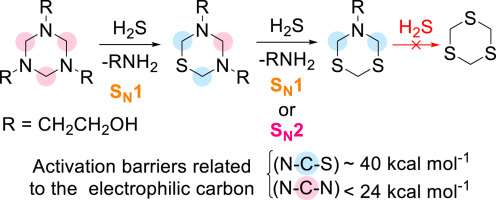
Hydrogen sulfide, H2S, stand as one of the major issues in petroleum industry. Aqueous solutions of triazines are the most utilized non-regenerative H2S scavenger. Although they present a theoretical capture capacity of 3 mol of H2S per mole of triazine, it effectively scavenges only 2 mol. Herein, we employed Density Functional Theory at CAM-B3LYP/6–311++G(2d,2p) level of calculation to rationalize the full mechanism for capture of H2S by hexahydro-1,3,5-triazines, in particular the reason why the reaction stops at the second equivalent of H2S. For the capture of the first equivalent of H2S, we found an unprecedented SN1 pathway to be less energetic than SN2, the most commonly accepted in literature. For capture of a second H2S molecule, however, both mechanisms are energetically similar. High barriers were found for the capture of the third molecule of H2S, related to the lower electrophilicity of the carbon atom bonded to nitrogen and sulfur atoms.
中文翻译:

DFT探索1,3,5-六氢三嗪清除H 2 S的机理
硫化氢,H 2 S,是石油工业的主要问题之一。三嗪的水溶液是最常用的非再生H 2 S清除剂。尽管它们的理论捕获容量为每摩尔三嗪3摩尔H 2 S,但它仅能有效清除2摩尔。在本文中,我们在CAM-B3LYP / 6–311 ++ G(2d,2p)的计算水平上采用了密度泛函理论,以合理化六氢-1,3,5-三嗪捕获H 2 S的完整机理,特别是反应在第二当量H 2 S处停止的原因。为了捕获第一当量H 2 S,我们发现前所未有的S N 1途径比S 2的能量更低N 2,在文学中最普遍接受的。但是,为了捕获第二个H 2 S分子,两种机理在能量上相似。发现捕获H 2 S的第三分子的障碍很高,这与键合到氮和硫原子上的碳原子的较低亲电性有关。


























 京公网安备 11010802027423号
京公网安备 11010802027423号