当前位置:
X-MOL 学术
›
J. Phys. Chem. Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Comparison of Exponential and Biexponential Models of the Unimolecular Decomposition Probability for the Hinshelwood–Lindemann Mechanism
The Journal of Physical Chemistry Letters ( IF 5.7 ) Pub Date : 2020-03-24 , DOI: 10.1021/acs.jpclett.0c00075 Philip W. Smith 1 , Bhumika Jayee 2 , William L. Hase 2
The Journal of Physical Chemistry Letters ( IF 5.7 ) Pub Date : 2020-03-24 , DOI: 10.1021/acs.jpclett.0c00075 Philip W. Smith 1 , Bhumika Jayee 2 , William L. Hase 2
Affiliation
|
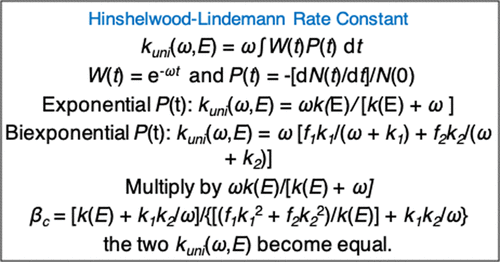
The traditional understanding is that the Hinshelwood–Lindemann mechanism for thermal unimolecular reactions, and the resulting unimolecular rate constant versus temperature and collision frequency ω (i.e., pressure), requires the Rice–Ramsperger–Kassel–Marcus (RRKM) rate constant k(E) to represent the unimolecular reaction of the excited molecule versus energy. RRKM theory assumes an exponential N(t)/N(0) population for the excited molecule versus time, with decay given by RRKM microcanonical k(E), and agreement between experimental and Hinshelwood–Lindemann thermal kinetics is then deemed to identify the unimolecular reactant as a RRKM molecule. However, recent calculations of the Hinshelwood–Lindemann rate constant kuni(ω,E) has brought this assumption into question. It was found that a biexponential N(t)/N(0), for intrinsic non-RRKM dynamics, gives a Hinshelwood–Lindemann kuni(ω,E) curve very similar to that of RRKM theory, which assumes exponential dynamics. The RRKM kuni(ω,E) curve was brought into agreement with the biexponential kuni(ω,E) by multiplying ω in the RRKM expression for kuni(ω,E) by an energy transfer efficiency factor βc. Such scaling is often done in fitting Hinshelwood–Lindemann–RRKM thermal kinetics to experiment. This agreement between the RRKM and non-RRKM curves for kuni(ω,E) was only obtained previously by scaling and fitting. In the work presented here, it is shown that if ω in the RRKM kuni(ω,E) is scaled by a βc factor there is analytic agreement with the non-RRKM kuni(ω,E). The expression for the value of βc is derived.
中文翻译:

Hinshelwood-Lindemann机理的单分子分解概率的指数模型和双指数模型的比较
传统的理解是,Hinshelwood-Lindemann热单分子反应机理以及由此产生的单分子速率常数与温度和碰撞频率ω(即压力)的关系,需要莱斯-兰斯珀格-卡塞尔-马库斯(RRKM)速率常数k(E)代表受激分子相对于能量的单分子反应。RRKM理论假设受激分子的N(t)/ N(0)指数随时间变化,衰减由RRKM微规范k(E),然后考虑实验和Hinshelwood-Lindemann热动力学之间的一致性,从而将单分子反应物鉴定为RRKM分子。但是,最近对Hinshelwood-Lindemann速率常数k uni(ω,E)的计算使这一假设受到质疑。结果发现,对于固有的非RRKM动力学,双指数N(t)/ N(0)给出了类似于假设指数动力学的RRKM理论的Hinshelwood–Lindemann k uni(ω,E)曲线。RRKM k uni(ω,E)曲线与双指数k一致UNI(ω,ê)由用于表达RRKM乘以ω ķ UNI(ω,ê通过能量转移效率因子)β Ç。通常通过将Hinshelwood–Lindemann–RRKM热动力学拟合为实验来完成这种缩放。k uni(ω,E)的RRKM和非RRKM曲线之间的一致性仅是通过缩放和拟合预先获得的。在工作中提出在这里,它表明,如果ω在RRKM ķ UNI(ω,ē)由缩放β ç因素存在与非RRKM解析协议ķ UNI(ω,ē)。对的值的表达式β Ç导出。
更新日期:2020-03-26
中文翻译:

Hinshelwood-Lindemann机理的单分子分解概率的指数模型和双指数模型的比较
传统的理解是,Hinshelwood-Lindemann热单分子反应机理以及由此产生的单分子速率常数与温度和碰撞频率ω(即压力)的关系,需要莱斯-兰斯珀格-卡塞尔-马库斯(RRKM)速率常数k(E)代表受激分子相对于能量的单分子反应。RRKM理论假设受激分子的N(t)/ N(0)指数随时间变化,衰减由RRKM微规范k(E),然后考虑实验和Hinshelwood-Lindemann热动力学之间的一致性,从而将单分子反应物鉴定为RRKM分子。但是,最近对Hinshelwood-Lindemann速率常数k uni(ω,E)的计算使这一假设受到质疑。结果发现,对于固有的非RRKM动力学,双指数N(t)/ N(0)给出了类似于假设指数动力学的RRKM理论的Hinshelwood–Lindemann k uni(ω,E)曲线。RRKM k uni(ω,E)曲线与双指数k一致UNI(ω,ê)由用于表达RRKM乘以ω ķ UNI(ω,ê通过能量转移效率因子)β Ç。通常通过将Hinshelwood–Lindemann–RRKM热动力学拟合为实验来完成这种缩放。k uni(ω,E)的RRKM和非RRKM曲线之间的一致性仅是通过缩放和拟合预先获得的。在工作中提出在这里,它表明,如果ω在RRKM ķ UNI(ω,ē)由缩放β ç因素存在与非RRKM解析协议ķ UNI(ω,ē)。对的值的表达式β Ç导出。



























 京公网安备 11010802027423号
京公网安备 11010802027423号