当前位置:
X-MOL 学术
›
ChemElectroChem
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Reduction Kinetics and Mass Transport of ZnO Single Entities on a Hg Ultramicroelectrode
ChemElectroChem ( IF 4 ) Pub Date : 2020-03-06 , DOI: 10.1002/celc.202000031 Nelum Karunathilake 1 , Salvador Gutierrez‐Portocarrero 1 , Pradeep Subedi 1 , Mario A. Alpuche‐Aviles 1
ChemElectroChem ( IF 4 ) Pub Date : 2020-03-06 , DOI: 10.1002/celc.202000031 Nelum Karunathilake 1 , Salvador Gutierrez‐Portocarrero 1 , Pradeep Subedi 1 , Mario A. Alpuche‐Aviles 1
Affiliation
|
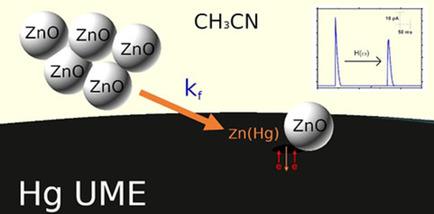
We discuss the electrolysis mechanism of colloidal ZnO NPs (10 nm diam.) in CH3CN. Stripping the preconcentrated Zn(Hg) allows quantification of the ZnO electrolyzed during stochastic interactions with the Hg surface. We model the mass transport taking the charged agglomerates of ZnO NPs as ionic species to calculate their migration and diffusional contributions. In unsupported suspensions, the mobility and positive zeta potential enhance transport towards the Hg UME. The NP electrolysis generates ionic species, increasing the migration rate and allowing lower detection limits compared to weakly supported suspensions, where the electrolyte modifies agglomerate charge and colloidal properties. We determine the kinetic constant (kf, in cm/s) for the ZnO reduction from the electrolysis transient model for destructive collisions of single entities, corrected for the potentiostat time constant. While most reduction events happen within 100 ms, the single entity model is consistent with mass transport studies over longer experimental times (1800 s).
中文翻译:

汞超微电极上ZnO单实体的还原动力学和质量输运
我们讨论了CH 3 CN中胶体ZnO NPs(直径为10 nm)的电解机理。剥离预浓缩的Zn(Hg)可以量化在与Hg表面的随机相互作用过程中电解的ZnO。我们以ZnO NPs的带电团聚体为离子物种,对物质的传输进行建模,以计算其迁移和扩散贡献。在无支撑的悬浮液中,迁移率和正zeta电位会增强向汞UME的转运。与弱支持的悬浮液相比,NP电解产生离子物质,从而增加了迁移速率并允许较低的检测限,在弱支持的悬浮液中,电解质会改变团聚体电荷和胶体性质。我们确定动力学常数(k f,单个实体的破坏性碰撞的电解瞬变模型中的ZnO还原量(以cm / s为单位),已针对恒电位仪时间常数进行了校正。尽管大多数还原事件都在100毫秒内发生,但单实体模型与更长的实验时间(1800 s)内的质量输运研究一致。
更新日期:2020-03-06
中文翻译:

汞超微电极上ZnO单实体的还原动力学和质量输运
我们讨论了CH 3 CN中胶体ZnO NPs(直径为10 nm)的电解机理。剥离预浓缩的Zn(Hg)可以量化在与Hg表面的随机相互作用过程中电解的ZnO。我们以ZnO NPs的带电团聚体为离子物种,对物质的传输进行建模,以计算其迁移和扩散贡献。在无支撑的悬浮液中,迁移率和正zeta电位会增强向汞UME的转运。与弱支持的悬浮液相比,NP电解产生离子物质,从而增加了迁移速率并允许较低的检测限,在弱支持的悬浮液中,电解质会改变团聚体电荷和胶体性质。我们确定动力学常数(k f,单个实体的破坏性碰撞的电解瞬变模型中的ZnO还原量(以cm / s为单位),已针对恒电位仪时间常数进行了校正。尽管大多数还原事件都在100毫秒内发生,但单实体模型与更长的实验时间(1800 s)内的质量输运研究一致。


























 京公网安备 11010802027423号
京公网安备 11010802027423号