当前位置:
X-MOL 学术
›
J. Chin. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Synthesis, antifungal and antibacterial activity of calix[4]arene‐based 1,3,4‐oxadiazole derivatives
Journal of the Chinese Chemical Society ( IF 1.8 ) Pub Date : 2020-03-06 , DOI: 10.1002/jccs.201900425 Zahra Dono Gezelbash 1 , Karim Akbari Dilmaghani 1
Journal of the Chinese Chemical Society ( IF 1.8 ) Pub Date : 2020-03-06 , DOI: 10.1002/jccs.201900425 Zahra Dono Gezelbash 1 , Karim Akbari Dilmaghani 1
Affiliation
|
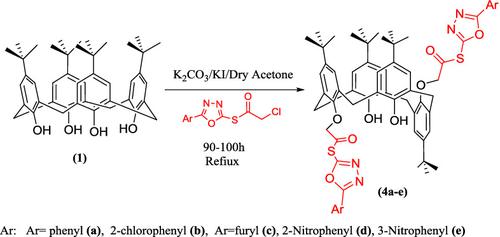
We describe the synthesis of some novel p ‐tert ‐butylcalix[4]arene‐based (5‐aryl‐1,3,4‐oxadiazol‐2‐yl)2‐chloroethanethioate derivatives (4a–e ). These compounds were synthesized by the reaction of tetra‐tert ‐butyl calix[4]arene (1 ) with (5‐aryl‐1,3,4‐oxadiazol‐2‐yl)2‐chloroethanethioate (3a–e ) in the presence of potassium carbonate as a weak base and dry acetone as the solvent. All the newly synthesized calix[4]arene derivatives were characterized by elemental analysis and various spectroscopic methods such as FT‐IR, 1H NMR,13C NMR, DEPT, and ESI‐MS. The synthesized compounds were tested in vitro for their antibacterial and antifungal activities against Escherichia coli and Aspergillus fumigates in comparison with enrofloxacin and amphotericin as reference drugs, which are normally used for treating such infections. The synthesized compounds showed different inhibition zones against the tested bacteria and fungi. Compound 4c was found to be most effective against A . fumigates , whereas compound 4e was found to be equally effective against E . coli and A. fumigates .
中文翻译:

杯[4]芳烃基1,3,4-恶二唑衍生物的合成,抗真菌和抗菌活性
我们描述了一些新颖的合成p -叔-butylcalix [4]芳烃基(5-芳基-1,3,4-恶二唑-2-基)-2- chloroethanethioate衍生物(4A-E )。这些化合物通过反应合成四-叔丁基杯[4]芳烃(1)与(5-芳基-1,3,4-恶二唑-2-基)-2- chloroethanethioate(3A-E在存在下)以碳酸钾为弱碱,以无水丙酮为溶剂。所有新合成的杯[4]芳烃衍生物均通过元素分析和各种光谱方法进行表征,例如FT-IR,1 H NMR,13 C NMR,DEPT和ESI-MS。合成的化合物在体外测试与恩诺沙星和两性霉素作参考药物相比,它们对大肠杆菌和熏蒸曲霉的抗菌和抗真菌活性较高,后者通常用于治疗此类感染。合成的化合物对测试的细菌和真菌显示出不同的抑制区。发现化合物4c对A最有效。熏蒸,而化合物4e被认为对E具有同样的作用。大肠杆菌和熏蒸曲霉。
更新日期:2020-03-06
中文翻译:

杯[4]芳烃基1,3,4-恶二唑衍生物的合成,抗真菌和抗菌活性
我们描述了一些新颖的合成p -叔-butylcalix [4]芳烃基(5-芳基-1,3,4-恶二唑-2-基)-2- chloroethanethioate衍生物(4A-E )。这些化合物通过反应合成四-叔丁基杯[4]芳烃(1)与(5-芳基-1,3,4-恶二唑-2-基)-2- chloroethanethioate(3A-E在存在下)以碳酸钾为弱碱,以无水丙酮为溶剂。所有新合成的杯[4]芳烃衍生物均通过元素分析和各种光谱方法进行表征,例如FT-IR,1 H NMR,13 C NMR,DEPT和ESI-MS。合成的化合物在体外测试与恩诺沙星和两性霉素作参考药物相比,它们对大肠杆菌和熏蒸曲霉的抗菌和抗真菌活性较高,后者通常用于治疗此类感染。合成的化合物对测试的细菌和真菌显示出不同的抑制区。发现化合物4c对A最有效。熏蒸,而化合物4e被认为对E具有同样的作用。大肠杆菌和熏蒸曲霉。



























 京公网安备 11010802027423号
京公网安备 11010802027423号