当前位置:
X-MOL 学术
›
Green Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
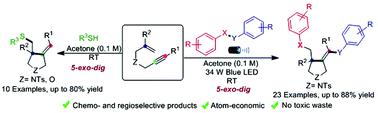
Alkene versus alkyne reactivity in unactivated 1,6-enynes: regio- and chemoselective radical cyclization with chalcogens under metal- and oxidant-free conditions
Green Chemistry ( IF 9.8 ) Pub Date : 2020-03-06 , DOI: 10.1039/d0gc00321b Mohana Reddy Mutra, Vishal Suresh Kudale, Jing Li, Wu-Hsun Tsai, Jeh-Jeng Wang
Green Chemistry ( IF 9.8 ) Pub Date : 2020-03-06 , DOI: 10.1039/d0gc00321b Mohana Reddy Mutra, Vishal Suresh Kudale, Jing Li, Wu-Hsun Tsai, Jeh-Jeng Wang
|
Herein, we have developed metal and oxidant-free visible light-promoted alkene vs. alkyne regio- and chemoselective radical cascade cyclization of electronically unbiased 1,6-enynes with chalcogens to synthesize substituted pyrrolidines bearing chalcogens. The reaction generated three new bonds, namely, C–SO2, C–C, and C–Se under extremely mild conditions. Furthermore, we achieved regio- and chemoselective mono-addition of aromatic thiophenols with unactivated 1,6-enynes. The key features of this protocol are broad substrate scope, environment-friendly conditions, operational simplicity, atom economy, and amenability to gram-scale synthesis. The mechanistic studies corroborate that the reaction proceeds via a radical pathway.
中文翻译:

未活化的1,6-炔烃中烯烃与炔烃的反应性:在无金属和无氧化剂的条件下用硫族元素对区域和化学选择性自由基进行环化
在这里,我们已经开发了无金属和无氧化剂的可见光促进的烯烃与炔烃的区域结合和化学选择性的自由基级联环化反应,将电子无偏的1,6-烯炔与硫族元素合成具有硫族元素的取代吡咯烷。该反应在极温和的条件下产生了三个新的键,即C–SO 2,CC和C–Se。此外,我们实现了芳香族苯酚与未活化的1,6-炔烃的区域和化学选择性单加成。该协议的关键特征是广泛的底物范围,环境友好的条件,操作简便,原子经济以及对克级合成的适应性。机理研究证实了反应是通过自由基途径进行的。
更新日期:2020-04-24
中文翻译:

未活化的1,6-炔烃中烯烃与炔烃的反应性:在无金属和无氧化剂的条件下用硫族元素对区域和化学选择性自由基进行环化
在这里,我们已经开发了无金属和无氧化剂的可见光促进的烯烃与炔烃的区域结合和化学选择性的自由基级联环化反应,将电子无偏的1,6-烯炔与硫族元素合成具有硫族元素的取代吡咯烷。该反应在极温和的条件下产生了三个新的键,即C–SO 2,CC和C–Se。此外,我们实现了芳香族苯酚与未活化的1,6-炔烃的区域和化学选择性单加成。该协议的关键特征是广泛的底物范围,环境友好的条件,操作简便,原子经济以及对克级合成的适应性。机理研究证实了反应是通过自由基途径进行的。


























 京公网安备 11010802027423号
京公网安备 11010802027423号