Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Adjuvant chemotherapy in upper tract urothelial carcinoma (the POUT trial): a phase 3, open-label, randomised controlled trial.
The Lancet ( IF 168.9 ) Pub Date : 2020-03-05 , DOI: 10.1016/s0140-6736(20)30415-3 Alison Birtle 1 , Mark Johnson 2 , John Chester 3 , Robert Jones 4 , David Dolling 5 , Richard T Bryan 6 , Christopher Harris 7 , Andrew Winterbottom 8 , Anthony Blacker 9 , James W F Catto 10 , Prabir Chakraborti 11 , Jenny L Donovan 12 , Paul Anthony Elliott 13 , Ann French 14 , Satinder Jagdev 15 , Benjamin Jenkins 5 , Francis Xavier Keeley 16 , Roger Kockelbergh 17 , Thomas Powles 18 , John Wagstaff 19 , Caroline Wilson 12 , Rachel Todd 5 , Rebecca Lewis 5 , Emma Hall 5
The Lancet ( IF 168.9 ) Pub Date : 2020-03-05 , DOI: 10.1016/s0140-6736(20)30415-3 Alison Birtle 1 , Mark Johnson 2 , John Chester 3 , Robert Jones 4 , David Dolling 5 , Richard T Bryan 6 , Christopher Harris 7 , Andrew Winterbottom 8 , Anthony Blacker 9 , James W F Catto 10 , Prabir Chakraborti 11 , Jenny L Donovan 12 , Paul Anthony Elliott 13 , Ann French 14 , Satinder Jagdev 15 , Benjamin Jenkins 5 , Francis Xavier Keeley 16 , Roger Kockelbergh 17 , Thomas Powles 18 , John Wagstaff 19 , Caroline Wilson 12 , Rachel Todd 5 , Rebecca Lewis 5 , Emma Hall 5
Affiliation
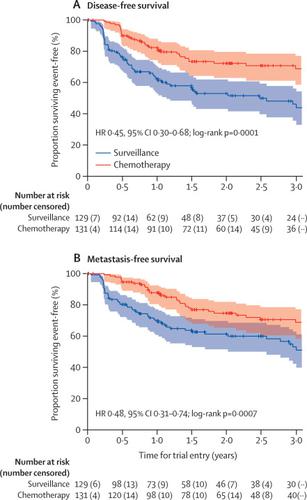
|
BACKGROUND
Urothelial carcinomas of the upper urinary tract (UTUCs) are rare, with poorer stage-for-stage prognosis than urothelial carcinomas of the urinary bladder. No international consensus exists on the benefit of adjuvant chemotherapy for patients with UTUCs after nephroureterectomy with curative intent. The POUT (Peri-Operative chemotherapy versus sUrveillance in upper Tract urothelial cancer) trial aimed to assess the efficacy of systemic platinum-based chemotherapy in patients with UTUCs.
METHODS
We did a phase 3, open-label, randomised controlled trial at 71 hospitals in the UK. We recruited patients with UTUC after nephroureterectomy staged as either pT2-T4 pN0-N3 M0 or pTany N1-3 M0. We randomly allocated participants centrally (1:1) to either surveillance or four 21-day cycles of chemotherapy, using a minimisation algorithm with a random element. Chemotherapy was either cisplatin (70 mg/m2) or carboplatin (area under the curve [AUC]4·5/AUC5, for glomerular filtration rate <50 mL/min only) administered intravenously on day 1 and gemcitabine (1000 mg/m2) administered intravenously on days 1 and 8; chemotherapy was initiated within 90 days of surgery. Follow-up included standard cystoscopic, radiological, and clinical assessments. The primary endpoint was disease-free survival analysed by intention to treat with a Peto-Haybittle stopping rule for (in)efficacy. The trial is registered with ClinicalTrials.gov, NCT01993979. A preplanned interim analysis met the efficacy criterion for early closure after recruitment of 261 participants.
FINDINGS
Between June 19, 2012, and Nov 8, 2017, we enrolled 261 participants from 57 of 71 open study sites. 132 patients were assigned chemotherapy and 129 surveillance. One participant allocated chemotherapy withdrew consent for data use after randomisation and was excluded from analyses. Adjuvant chemotherapy significantly improved disease-free survival (hazard ratio 0·45, 95% CI 0·30-0·68; p=0·0001) at a median follow-up of 30·3 months (IQR 18·0-47·5). 3-year event-free estimates were 71% (95% CI 61-78) and 46% (36-56) for chemotherapy and surveillance, respectively. 55 (44%) of 126 participants who started chemotherapy had acute grade 3 or worse treatment-emergent adverse events, which accorded with frequently reported events for the chemotherapy regimen. Five (4%) of 129 patients managed by surveillance had acute grade 3 or worse emergent adverse events. No treatment-related deaths were reported.
INTERPRETATION
Gemcitabine-platinum combination chemotherapy initiated within 90 days after nephroureterectomy significantly improved disease-free survival in patients with locally advanced UTUC. Adjuvant platinum-based chemotherapy should be considered a new standard of care after nephroureterectomy for this patient population.
FUNDING
Cancer Research UK.
中文翻译:

上尿路尿路上皮癌的辅助化疗(POUT 试验):一项 3 期、开放标签、随机对照试验。
背景 上尿路尿路上皮癌 (UTUC) 很少见,分期预后比膀胱尿路上皮癌差。对于以治愈为目的的肾输尿管切除术后 UTUC 患者的辅助化疗的益处,尚无国际共识。POUT(围手术期化疗与上尿路尿路上皮癌监测)试验旨在评估全身铂类化疗对 UTUC 患者的疗效。方法 我们在英国的 71 家医院进行了一项 3 期、开放标签、随机对照试验。我们招募了肾输尿管切除术后 UTUC 患者,分期为 pT2-T4 pN0-N3 M0 或 pTany N1-3 M0。我们将参与者集中随机分配 (1:1) 接受监测或四个 21 天周期的化疗,使用具有随机元素的最小化算法。化疗是在第 1 天静脉内给予顺铂 (70 mg/m2) 或卡铂(曲线下面积 [AUC]4·5/AUC5,仅肾小球滤过率 <50 mL/min)和吉西他滨 (1000 mg/m2)在第 1 天和第 8 天静脉内给药;化疗在手术后 90 天内开始。随访包括标准的膀胱镜检查、放射学和临床评估。主要终点是无病生存期,通过使用 Peto-Haybittle 停止规则(无效)的意向治疗进行分析。该试验已在 ClinicalTrials.gov 注册,NCT01993979。在招募 261 名参与者后,预先计划的中期分析符合提前关闭的疗效标准。2012 年 6 月 19 日至 2017 年 11 月 8 日期间的调查结果,我们从 71 个开放研究中心中的 57 个中心招募了 261 名参与者。132 名患者接受化疗,129 名患者接受监测。分配化疗的一名参与者在随机分组后撤回了对数据使用的同意,并被排除在分析之外。在中位随访 30·3 个月 (IQR 18·0-47) 时,辅助化疗显着改善了无病生存期(风险比 0·45,95% CI 0·30-0·68;p=0·0001) ·5). 化疗和监测的 3 年无事件估计分别为 71% (95% CI 61-78) 和 46% (36-56)。开始化疗的 126 名参与者中有 55 名 (44%) 出现急性 3 级或更严重的治疗突发不良事件,这与化疗方案中经常报告的事件一致。接受监测的 129 名患者中有 5 名 (4%) 出现急性 3 级或更严重的紧急不良事件。没有报告与治疗相关的死亡。解释 肾输尿管切除术后 90 天内开始吉西他滨-铂联合化疗可显着改善局部晚期 UTUC 患者的无病生存期。基于铂类的辅助化疗应被视为该患者群体肾输尿管切除术后的新标准治疗。资助英国癌症研究。
更新日期:2020-04-22
中文翻译:

上尿路尿路上皮癌的辅助化疗(POUT 试验):一项 3 期、开放标签、随机对照试验。
背景 上尿路尿路上皮癌 (UTUC) 很少见,分期预后比膀胱尿路上皮癌差。对于以治愈为目的的肾输尿管切除术后 UTUC 患者的辅助化疗的益处,尚无国际共识。POUT(围手术期化疗与上尿路尿路上皮癌监测)试验旨在评估全身铂类化疗对 UTUC 患者的疗效。方法 我们在英国的 71 家医院进行了一项 3 期、开放标签、随机对照试验。我们招募了肾输尿管切除术后 UTUC 患者,分期为 pT2-T4 pN0-N3 M0 或 pTany N1-3 M0。我们将参与者集中随机分配 (1:1) 接受监测或四个 21 天周期的化疗,使用具有随机元素的最小化算法。化疗是在第 1 天静脉内给予顺铂 (70 mg/m2) 或卡铂(曲线下面积 [AUC]4·5/AUC5,仅肾小球滤过率 <50 mL/min)和吉西他滨 (1000 mg/m2)在第 1 天和第 8 天静脉内给药;化疗在手术后 90 天内开始。随访包括标准的膀胱镜检查、放射学和临床评估。主要终点是无病生存期,通过使用 Peto-Haybittle 停止规则(无效)的意向治疗进行分析。该试验已在 ClinicalTrials.gov 注册,NCT01993979。在招募 261 名参与者后,预先计划的中期分析符合提前关闭的疗效标准。2012 年 6 月 19 日至 2017 年 11 月 8 日期间的调查结果,我们从 71 个开放研究中心中的 57 个中心招募了 261 名参与者。132 名患者接受化疗,129 名患者接受监测。分配化疗的一名参与者在随机分组后撤回了对数据使用的同意,并被排除在分析之外。在中位随访 30·3 个月 (IQR 18·0-47) 时,辅助化疗显着改善了无病生存期(风险比 0·45,95% CI 0·30-0·68;p=0·0001) ·5). 化疗和监测的 3 年无事件估计分别为 71% (95% CI 61-78) 和 46% (36-56)。开始化疗的 126 名参与者中有 55 名 (44%) 出现急性 3 级或更严重的治疗突发不良事件,这与化疗方案中经常报告的事件一致。接受监测的 129 名患者中有 5 名 (4%) 出现急性 3 级或更严重的紧急不良事件。没有报告与治疗相关的死亡。解释 肾输尿管切除术后 90 天内开始吉西他滨-铂联合化疗可显着改善局部晚期 UTUC 患者的无病生存期。基于铂类的辅助化疗应被视为该患者群体肾输尿管切除术后的新标准治疗。资助英国癌症研究。


























 京公网安备 11010802027423号
京公网安备 11010802027423号