Gastroenterology ( IF 29.4 ) Pub Date : 2020-03-06 , DOI: 10.1053/j.gastro.2020.02.058 Michel Bazinet 1 , Victor Pântea 2 , Gheorghe Placinta 2 , Iurie Moscalu 3 , Valentin Cebotarescu 2 , Lilia Cojuhari 2 , Pavlina Jimbei 4 , Liviu Iarovoi 2 , Valentina Smesnoi 4 , Tatiana Musteata 4 , Alina Jucov 5 , Ulf Dittmer 6 , Adalbert Krawczyk 7 , Andrew Vaillant 1
|
Background & Aims
Nucleic acid polymers (NAPs) inhibit assembly and secretion of hepatitis B virus (HBV) subviral particles. We performed an open-label, phase 2 study of the safety and efficacy of the NAPs REP 2139 or REP 2165 combined with tenofovir disoproxil fumarate (TDF) and pegylated interferon alfa-2a (pegIFN) in patients with chronic HBV infection who were negative for hepatitis B e antigen.
Methods
Following 24 weeks TDF therapy, 40 patients were randomly assigned to groups that received 48 weeks of experimental therapy (TDF + pegIFN + REP 2139-Mg or REP 2165-Mg) or 24 weeks of control therapy (TDF + pegIFN) followed by 48 weeks of experimental therapy. Patients were then followed for a treatment-free period of 48 weeks. Primary outcomes were the safety and tolerability of REP 2139-Mg or REP 2165-Mg in combination with TDF + pegIFN compared with TDF + pegIFN alone through the first 48 weeks of therapy and subsequently throughout 48 weeks of NAP-based combination therapy (treatment weeks 24–72 in the experimental group and weeks 48–96 in the control group). Secondary outcomes were reductions in hepatitis B surface antigen (HBsAg) in control and experimental groups over the first 48 weeks of the study and throughout 48 weeks of combination therapy and virologic control (HBsAg positive, HBV DNA below 2000 IU/mL, normal level of alanine aminotransferase) or functional cure (HBsAg below 0.05 IU/mL, HBV DNA target not detected, normal level of alanine aminotransferase) after removal of all therapy.
Results
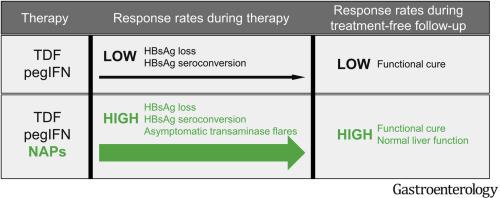
Levels of HBsAg, anti-HBs, and HBV DNA did not differ significantly between the groups given REP 2139 vs REP 2165. PegIFN-induced thrombocytopenia (P = .299 vs controls) and neutropenia (P = .112 vs controls) were unaffected by NAPs (REP 2139 vs REP 2165). Increases in levels of transaminases were significantly more frequent (P < .001 vs controls) and greater (P = .002 vs controls) in the NAP groups (but did not produce symptoms), correlated with initial decrease in HBsAg, and normalized during therapy and follow-up. During the first 24 weeks of TDF and pegIFN administration, significantly higher proportions of patients in NAP groups had decreases in HBsAg to below 1 IU/mL (P < .001 vs control) and HBsAg seroconversion (P = .046 vs control). At the time patients completed the TDF + pegIFN + NAP regimen, HBsAg levels were 0.05 IU/mL or lower in 24/40 participants (all with seroconversion up to 233,055 mIU/mL). During 48 weeks of treatment-free follow-up, virologic control persisted in 13 of 40 participants (2 lost to follow-up after 24 weeks), whereas functional cure persisted in 14 of 40 participants (all completing 48 weeks of follow-up) with persistent HBsAg seroconversion. One participant had a viral rebound during follow-up with hepatic decompensation and was placed on TDF therapy.
Conclusions
In a phase 2 randomized trial, we found that addition of NAPs to TDF + pegIFN did not alter tolerability and significantly increased rates of HBsAg loss and HBsAg seroconversion during therapy and functional cure after therapy. Clinicaltrials.gov no: NCT02565719.
中文翻译:

初治Nucleos(t)ide治疗的慢性HBV感染患者48周REP 2139或REP 2165,替诺福韦二甲酚和聚乙二醇化干扰素Alfa-2a的安全性和有效性。
背景与目标
核酸聚合物(NAP)抑制乙型肝炎病毒(HBV)亚病毒颗粒的组装和分泌。我们对NAP阴性的NAP REP 2139或REP 2165联合富马酸替诺福韦酯(TDF)和聚乙二醇化干扰素alfa-2a(pegIFN)的安全性和有效性进行了2期安全性和开放性研究乙型肝炎e抗原。
方法
TDF治疗24周后,将40例患者随机分为接受48周实验治疗(TDF + pegIFN + REP 2139-Mg或REP 2165-Mg)或24周对照治疗(TDF + pegIFN)的组,随后48周实验疗法。然后,对患者进行48周的免费治疗。主要结局是在治疗的前48周以及随后整个基于NAP的联合治疗的整个48周(治疗周)中,REP 2139-Mg或REP 2165-Mg与TDF + pegIFN联合使用与单独使用TDF + pegIFN相比,其安全性和耐受性实验组为24-72周,对照组为48-96周)。
结果
给予REP 2139和REP 2165的组之间的HBsAg,抗HBs和HBV DNA的水平没有显着差异。PegIFN诱导的血小板减少症(P = .299对对照)和中性粒细胞减少症(P = .112对对照)不受影响。 NAP(REP 2139与REP 2165)。在 NAP组中转氨酶水平的升高更为频繁(P <.001 vs对照),而更高(P = .002 vs对照)(但未产生症状),与最初的HBsAg降低相关,并在治疗期间恢复正常和跟进。在TDF和pegIFN给药的最初24周内,NAP组患者的HBsAg降低至1 IU / mL以下的比例显着更高(P<0.001 vs对照)和HBsAg血清转化(P = .046 vs对照)。在患者完成TDF + pegIFN + NAP方案时,24/40名参与者的HBsAg水平为0.05 IU / mL或更低(所有患者的血清转化率高达233,055 mIU / mL)。在48周的无治疗随访中,40名参与者中有13名坚持了病毒学控制(24周后失去了2名随访),而40名参与者中有14名坚持了功能性治愈(全部完成了48周的随访)持续存在HBsAg血清转化。一名参与者在进行肝代偿失调的随访过程中出现病毒反弹,并接受了TDF治疗。
结论
在一项2期随机试验中,我们发现在TDF + pegIFN中添加NAP不会改变耐受性,并且在治疗期间和治疗后功能治愈期间HBsAg丢失和HBsAg血清转化的比率显着增加。Clinicaltrials.gov编号:NCT02565719。



























 京公网安备 11010802027423号
京公网安备 11010802027423号