当前位置:
X-MOL 学术
›
Nanomed. Nanotech. Biol. Med.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Photoactivation of sulfonated polyplexes enables localized gene silencing by DsiRNA in breast cancer cells.
Nanomedicine: Nanotechnology, Biology and Medicine ( IF 5.4 ) Pub Date : 2020-03-06 , DOI: 10.1016/j.nano.2020.102176 Anu Puri 1 , Mathias Viard 2 , Paul Zakrevsky 1 , Serena Zampino 1 , Arabella Chen 1 , Camryn Isemann 1 , Sohaib Alvi 1 , Jeff Clogston 3 , Upendra Chitgupi 4 , Jonathan F Lovell 4 , Bruce A Shapiro 1
Nanomedicine: Nanotechnology, Biology and Medicine ( IF 5.4 ) Pub Date : 2020-03-06 , DOI: 10.1016/j.nano.2020.102176 Anu Puri 1 , Mathias Viard 2 , Paul Zakrevsky 1 , Serena Zampino 1 , Arabella Chen 1 , Camryn Isemann 1 , Sohaib Alvi 1 , Jeff Clogston 3 , Upendra Chitgupi 4 , Jonathan F Lovell 4 , Bruce A Shapiro 1
Affiliation
|
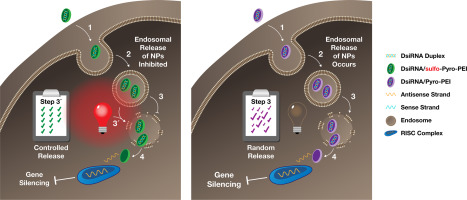
Translation potential of RNA interference nanotherapeutics remains challenging due to in vivo off-target effects and poor endosomal escape. Here, we developed novel polyplexes for controlled intracellular delivery of dicer substrate siRNA, using a light activation approach. Sulfonated polyethylenimines covalently linked to pyropheophorbide-α for photoactivation and bearing modified amines (sulfo-pyro-PEI) for regulated endosomal escape were investigated. Gene knock-down by the polymer-complexed DsiRNA duplexes (siRNA-NPs) was monitored in breast cancer cells. Surprisingly, sulfo-pyro-PEI/siRNA-NPs failed to downregulate the PLK1 or eGFP proteins. However, photoactivation of these cell associated-polyplexes with a 661-nm laser clearly restored knock-down of both proteins. In contrast, protein down-regulation by non-sulfonated pyro-PEI/siRNA-NPs occurred without any laser treatments, indicating cytoplasmic disposition of DsiRNA followed a common intracellular release mechanism. Therefore, sulfonated pyro-PEI holds potential as a unique trap and release light-controlled delivery platform for on-demand gene silencing bearing minimal off target effects.
中文翻译:

磺化多聚体的光活化使得DsiRNA在乳腺癌细胞中能够实现局部基因沉默。
由于体内脱靶效应和不良的内体逃逸,RNA干扰纳米疗法的翻译潜力仍然具有挑战性。在这里,我们使用光激活方法开发了新型多聚体,用于控制切丁酶底物siRNA的细胞内递送。研究了与焦脱镁叶绿素-α共价连接的磺化聚乙烯亚胺用于光活化,并带有修饰的胺(磺基-焦磷酸-PEI)用于调节内体逃逸。在乳腺癌细胞中监测了聚合物复合的DsiRNA双链体(siRNA-NPs)的基因敲低。令人惊讶的是,磺基-焦磷酸-PEI / siRNA-NPs无法下调PLK1或eGFP蛋白。但是,用661 nm激光对这些细胞相关多聚体的光活化作用明显恢复了两种蛋白的敲低。相反,未经磺化的pyro-PEI / siRNA-NPs引起的蛋白下调未经过任何激光处理,表明DsiRNA的细胞质沉积遵循常见的细胞内释放机制。因此,磺化吡咯-PEI具有潜在的独特捕获和释放光控传递平台的功能,可按需沉默基因,从而使脱靶效应降至最低。
更新日期:2020-03-06
中文翻译:

磺化多聚体的光活化使得DsiRNA在乳腺癌细胞中能够实现局部基因沉默。
由于体内脱靶效应和不良的内体逃逸,RNA干扰纳米疗法的翻译潜力仍然具有挑战性。在这里,我们使用光激活方法开发了新型多聚体,用于控制切丁酶底物siRNA的细胞内递送。研究了与焦脱镁叶绿素-α共价连接的磺化聚乙烯亚胺用于光活化,并带有修饰的胺(磺基-焦磷酸-PEI)用于调节内体逃逸。在乳腺癌细胞中监测了聚合物复合的DsiRNA双链体(siRNA-NPs)的基因敲低。令人惊讶的是,磺基-焦磷酸-PEI / siRNA-NPs无法下调PLK1或eGFP蛋白。但是,用661 nm激光对这些细胞相关多聚体的光活化作用明显恢复了两种蛋白的敲低。相反,未经磺化的pyro-PEI / siRNA-NPs引起的蛋白下调未经过任何激光处理,表明DsiRNA的细胞质沉积遵循常见的细胞内释放机制。因此,磺化吡咯-PEI具有潜在的独特捕获和释放光控传递平台的功能,可按需沉默基因,从而使脱靶效应降至最低。



























 京公网安备 11010802027423号
京公网安备 11010802027423号