当前位置:
X-MOL 学术
›
Biotechnol. Appl. Bioc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Artificial iron hydrogenase made by covalent grafting of Knölker's complex into xylanase: Application in asymmetric hydrogenation of an aryl ketone in water.
Biotechnology and Applied Biochemistry ( IF 2.8 ) Pub Date : 2020-03-05 , DOI: 10.1002/bab.1906 Kalani Kariyawasam 1 , Wadih Ghattas 1 , Yossef López De Los Santos 2 , Nicolas Doucet 2 , Sylvain Gaillard 3 , Jean-Luc Renaud 3 , Frédéric Avenier 1 , Jean-Pierre Mahy 1 , Rémy Ricoux 1
Biotechnology and Applied Biochemistry ( IF 2.8 ) Pub Date : 2020-03-05 , DOI: 10.1002/bab.1906 Kalani Kariyawasam 1 , Wadih Ghattas 1 , Yossef López De Los Santos 2 , Nicolas Doucet 2 , Sylvain Gaillard 3 , Jean-Luc Renaud 3 , Frédéric Avenier 1 , Jean-Pierre Mahy 1 , Rémy Ricoux 1
Affiliation
|
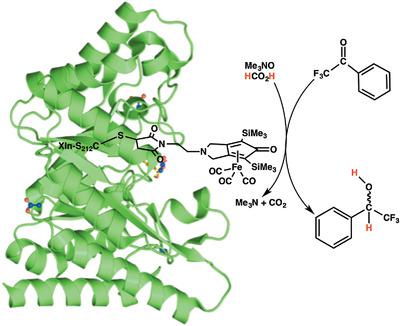
We report a new artificial hydrogenase made by covalent anchoring of the iron Knölker's complex to a xylanase S212C variant. This artificial metalloenzyme was found to be able to catalyze efficiently the transfer hydrogenation of the benchmark substrate trifluoroacetophenone by sodium formate in water, yielding the corresponding secondary alcohol as a racemic. The reaction proceeded more than threefold faster with the XlnS212CK biohybrid than with the Knölker's complex alone. In addition, efficient conversion of trifluoroacetophenone to its corresponding alcohol was reached within 60 H with XlnS212CK, whereas a ≈2.5‐fold lower conversion was observed with Knölker's complex alone as catalyst. Moreover, the data were rationalized with a computational strategy suggesting the key factors of the selectivity. These results suggested that the Knölker's complex was most likely flexible and could experience free rotational reorientation within the active‐site pocket of Xln A, allowing it to access the subsite pocket populated by trifluoroacetophenone.
中文翻译:

通过将Knölker配合物共价接枝到木聚糖酶中制成的人工铁氢化酶:在水中芳基酮的不对称氢化中的应用。
我们报告了一种新的人工加氢酶,该酶由共价锚定铁克诺尔克氏复合物至木聚糖酶S212C变体制成。发现该人造金属酶能够有效地催化基准甲酸酯基三氟苯乙酮在水中被甲酸钠转移氢化,产生相应的仲醇为外消旋体。XlnS212CK生物杂交体的反应进行速度比单独的Knölker复合物快三倍以上。此外,使用XlnS212CK可以在60 H内将三氟苯乙酮有效转化为相应的醇,而仅使用Knölker配合物作为催化剂,转化率降低了约2.5倍。此外,通过计算策略合理化数据,表明了选择性的关键因素。
更新日期:2020-03-05
中文翻译:

通过将Knölker配合物共价接枝到木聚糖酶中制成的人工铁氢化酶:在水中芳基酮的不对称氢化中的应用。
我们报告了一种新的人工加氢酶,该酶由共价锚定铁克诺尔克氏复合物至木聚糖酶S212C变体制成。发现该人造金属酶能够有效地催化基准甲酸酯基三氟苯乙酮在水中被甲酸钠转移氢化,产生相应的仲醇为外消旋体。XlnS212CK生物杂交体的反应进行速度比单独的Knölker复合物快三倍以上。此外,使用XlnS212CK可以在60 H内将三氟苯乙酮有效转化为相应的醇,而仅使用Knölker配合物作为催化剂,转化率降低了约2.5倍。此外,通过计算策略合理化数据,表明了选择性的关键因素。


























 京公网安备 11010802027423号
京公网安备 11010802027423号