PLoS Pathogens ( IF 6.7 ) Pub Date : 2020-03-05 , DOI: 10.1371/journal.ppat.1008296 Jessica R Doll 1 , Kasper Hoebe 2 , Richard L Thompson 1 , Nancy M Sawtell 2
|
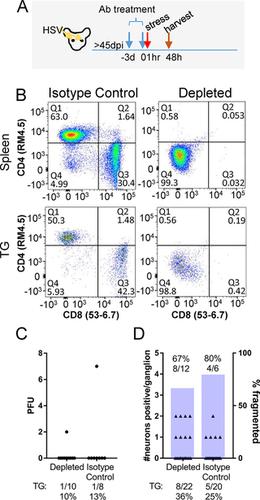
A fundamental question in herpes simplex virus (HSV) pathogenesis is the consequence of viral reactivation to the neuron. Evidence supporting both post-reactivation survival and demise is published. The exceedingly rare nature of this event at the neuronal level in the sensory ganglion has limited direct examination of this important question. In this study, an in-depth in vivo analysis of the resolution of reactivation was undertaken. Latently infected C57BL/6 mice were induced to reactivate in vivo by hyperthermic stress. Infectious virus was detected in a high percentage (60–80%) of the trigeminal ganglia from these mice at 20 hours post-reactivation stimulus, but declined by 48 hours post-stimulus (0–13%). With increasing time post-reactivation stimulus, the percentage of reactivating neurons surrounded by a cellular cuff increased, which correlated with a decrease in detectable infectious virus and number of viral protein positive neurons. Importantly, in addition to intact viral protein positive neurons, fragmented viral protein positive neurons morphologically consistent with apoptotic bodies and containing cleaved caspase-3 were detected. The frequency of this phenotype increased through time post-reactivation. These fragmented neurons were surrounded by Iba1+ cells, consistent with phagocytic removal of dead neurons. Evidence of neuronal destruction post-reactivation prompted re-examination of the previously reported non-cytolytic role of T cells in controlling reactivation. Latently infected mice were treated with anti-CD4/CD8 antibodies prior to induced reactivation. Neither infectious virus titers nor neuronal fragmentation were altered. In contrast, when viral DNA replication was blocked during reactivation, fragmentation was not observed even though viral proteins were expressed. Our data demonstrate that at least a portion of reactivating neurons are destroyed. Although no evidence for direct T cell mediated antigen recognition in this process was apparent, inhibition of viral DNA replication blocked neuronal fragmentation. These unexpected findings raise new questions about the resolution of HSV reactivation in the host nervous system.
中文翻译:

体内单纯疱疹病毒再激活的解决导致神经元破坏。
单纯疱疹病毒(HSV)发病机理中的一个基本问题是病毒重新激活神经元的结果。支持再激活后存活和死亡的证据已经发表。该事件在感觉神经节的神经元水平上极为罕见,限制了对该重要问题的直接检查。在这项研究中,深入体内对重新激活的解决方案进行了分析。潜伏感染的C57BL / 6小鼠在体内被高温应激诱导重新激活。在重新激活刺激后20小时,这些小鼠的三叉神经节中检出感染性病毒的比例很高(60–80%),但在刺激后48小时下降了(0–13%)。随着激活后刺激时间的增加,被细胞套囊包围的激活神经元的百分比增加,这与可检测的传染性病毒和病毒蛋白阳性神经元数量的减少有关。重要的是,除了完整的病毒蛋白阳性神经元外,还检测到碎片化的病毒蛋白阳性神经元的形态与凋亡小体一致,并含有裂解的caspase-3。该表型的频率通过重新激活后的时间增加。+细胞,与吞噬细胞去除死亡的神经元一致。激活后神经元破坏的证据促使人们重新检查先前报道的T细胞在控制激活中的非溶细胞作用。在诱导的重新激活之前,用抗CD4 / CD8抗体治疗潜伏感染的小鼠。传染性病毒滴度和神经元片段均未改变。相反,当病毒DNA复制在再激活过程中被阻断时,即使表达了病毒蛋白也未观察到片段化。我们的数据表明,至少一部分激活神经元被破坏。尽管在此过程中尚无直接T细胞介导的抗原识别的证据,但抑制病毒DNA复制可阻止神经元断裂。


























 京公网安备 11010802027423号
京公网安备 11010802027423号