当前位置:
X-MOL 学术
›
Colloids Surf. B Biointerfaces
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Interaction of curium(III) with surface-layer proteins from Lysinibacillus sphaericus JG-A12.
Colloids and Surfaces B: Biointerfaces ( IF 5.8 ) Pub Date : 2020-03-05 , DOI: 10.1016/j.colsurfb.2020.110950 Henry Moll 1 , Falk Lehmann 1 , Johannes Raff 1
Colloids and Surfaces B: Biointerfaces ( IF 5.8 ) Pub Date : 2020-03-05 , DOI: 10.1016/j.colsurfb.2020.110950 Henry Moll 1 , Falk Lehmann 1 , Johannes Raff 1
Affiliation
|
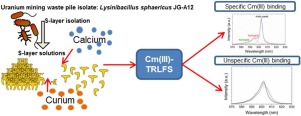
Trivalent actinides such as Cm(III) are able to occupy natural Ca(II) binding sites in biological systems. For this investigation, we studied the formation of aqueous Cm(III) complexes with S-layer proteins by time-resolved laser-induced fluorescence spectroscopy (TRLFS). S-layer proteins serve as protective biointerfaces in bacteria and archaea against the surrounding solution. Experimental assays were performed at a fixed total concentration of Cm(III) (0.88 μM) using an S-layer protein (5 g/L / 39.6 μM) at varying pH levels (2.0-9.0), as well as several types of S-layer proteins of L. sphaericus JG-A12. Based on resulting luminescence spectra and lifetime data, specific and unspecific binding sites could be distinguished. Notably, specific Cm(III) binding to S-layer proteins was confirmed by the appearance of a sharp emission band at 602.5 nm, combined with a long lifetime of 310 μs. The high affinity of these specific binding sites was also verified using competing EDTA, wherein only a high EDTA concentration (40 μM) could efficiently remove Cm(III) from S-layer proteins.
中文翻译:

ium(III)与球形芽孢杆菌JG-A12的表面层蛋白的相互作用。
三价act系元素(例如Cm(III))能够占据生物系统中的天然Ca(II)结合位点。为了进行这项研究,我们研究了时间分辨的激光诱导荧光光谱法(TRLFS)与S层蛋白形成的Cm(III)水溶液的形成。S层蛋白可作为细菌和古细菌对抗周围溶液的保护性生物界面。使用固定在Cm(III)总浓度(0.88μM)下的S-层蛋白(5 g / L / 39.6μM)在不同的pH值(2.0-9.0)以及几种类型的S上进行实验分析球形乳杆菌JG-A12的层蛋白。基于所得的发光光谱和寿命数据,可以区分特异性和非特异性结合位点。值得注意的是,通过在602.5 nm处出现清晰的发射带,证实了与S层蛋白结合的特定Cm(III),结合了310μs的长寿命。还使用竞争性EDTA验证了这些特异性结合位点的高亲和力,其中只有高EDTA浓度(40μM)才能有效地从S层蛋白中去除Cm(III)。
更新日期:2020-03-05
中文翻译:

ium(III)与球形芽孢杆菌JG-A12的表面层蛋白的相互作用。
三价act系元素(例如Cm(III))能够占据生物系统中的天然Ca(II)结合位点。为了进行这项研究,我们研究了时间分辨的激光诱导荧光光谱法(TRLFS)与S层蛋白形成的Cm(III)水溶液的形成。S层蛋白可作为细菌和古细菌对抗周围溶液的保护性生物界面。使用固定在Cm(III)总浓度(0.88μM)下的S-层蛋白(5 g / L / 39.6μM)在不同的pH值(2.0-9.0)以及几种类型的S上进行实验分析球形乳杆菌JG-A12的层蛋白。基于所得的发光光谱和寿命数据,可以区分特异性和非特异性结合位点。值得注意的是,通过在602.5 nm处出现清晰的发射带,证实了与S层蛋白结合的特定Cm(III),结合了310μs的长寿命。还使用竞争性EDTA验证了这些特异性结合位点的高亲和力,其中只有高EDTA浓度(40μM)才能有效地从S层蛋白中去除Cm(III)。



























 京公网安备 11010802027423号
京公网安备 11010802027423号