当前位置:
X-MOL 学术
›
ACS Med. Chem. Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Ahp-Cyclodepsipeptide Inhibitors of Elastase: Lyngbyastatin 7 Stability, Scalable Synthesis, and Focused Library Analysis.
ACS Medicinal Chemistry Letters ( IF 4.2 ) Pub Date : 2020-03-04 , DOI: 10.1021/acsmedchemlett.9b00473 Danmeng Luo 1, 2 , Hendrik Luesch 1, 2
ACS Medicinal Chemistry Letters ( IF 4.2 ) Pub Date : 2020-03-04 , DOI: 10.1021/acsmedchemlett.9b00473 Danmeng Luo 1, 2 , Hendrik Luesch 1, 2
Affiliation
|
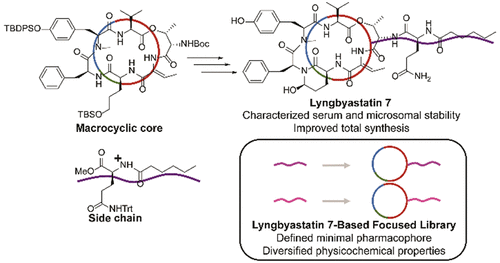
Due to the potency and selectivity of lyngbyastatin 7 in inhibiting neutrophil elastase, a serine protease involved in numerous diseases, this cyclodepsipeptide was considered as a promising lead and subjected to further developmental studies. Lyngbyastatin 7 displayed a favorable serum and microsomal stability profile. The large-scale synthesis of key building blocks was performed on gram scale with improved yields and simplified purification procedures. To tailor the complex structure, define the minimal pharmacophore, and modulate the physicochemical properties of the lead scaffold, the first pilot library of analogues was designed and synthesized for structure-activity relationship studies. We uncovered the essential role of the side chain, indicating that the minimal structural requirements for elastase inhibition extended beyond the 3-amino-6-hydroxy-2-piperidone (Ahp) and 2-aminobutenoic acid (Abu) moieties conventionally known to convey antiprotease activity and elastase selectivity, respectively. Our studies will facilitate the design and development of this class of elastase inhibitors.
中文翻译:

弹性蛋白酶的Ahp-Cyclodepsipeptide抑制剂:Lyngbyastatin 7稳定性,可扩展合成和集中库分析。
由于lyngbyastatin 7抑制嗜中性粒细胞弹性蛋白酶(一种参与多种疾病的丝氨酸蛋白酶)的效力和选择性,该环二肽被认为是有前途的先导,并受到了进一步的开发研究。Lyngbyastatin 7显示出良好的血清和微粒体稳定性。关键组成部分的大规模合成以克级进行,具有提高的产率和简化的纯化程序。为了定制复杂的结构,定义最小的药效基团,并调节铅支架的理化性质,设计并合成了第一个类似物先导库用于结构-活性关系研究。我们发现了侧链的重要作用,这表明对弹性蛋白酶抑制作用的最低结构要求超出了通常已知分别传达抗蛋白酶活性和弹性蛋白酶选择性的3-氨基-6-羟基-2-哌啶酮(Ahp)和2-氨基丁烯酸(Abu)部分。我们的研究将促进此类弹性蛋白酶抑制剂的设计和开发。
更新日期:2020-04-23
中文翻译:

弹性蛋白酶的Ahp-Cyclodepsipeptide抑制剂:Lyngbyastatin 7稳定性,可扩展合成和集中库分析。
由于lyngbyastatin 7抑制嗜中性粒细胞弹性蛋白酶(一种参与多种疾病的丝氨酸蛋白酶)的效力和选择性,该环二肽被认为是有前途的先导,并受到了进一步的开发研究。Lyngbyastatin 7显示出良好的血清和微粒体稳定性。关键组成部分的大规模合成以克级进行,具有提高的产率和简化的纯化程序。为了定制复杂的结构,定义最小的药效基团,并调节铅支架的理化性质,设计并合成了第一个类似物先导库用于结构-活性关系研究。我们发现了侧链的重要作用,这表明对弹性蛋白酶抑制作用的最低结构要求超出了通常已知分别传达抗蛋白酶活性和弹性蛋白酶选择性的3-氨基-6-羟基-2-哌啶酮(Ahp)和2-氨基丁烯酸(Abu)部分。我们的研究将促进此类弹性蛋白酶抑制剂的设计和开发。


























 京公网安备 11010802027423号
京公网安备 11010802027423号