Mini-Reviews in Medicinal Chemistry ( IF 3.8 ) Pub Date : 2020-06-30 , DOI: 10.2174/1389557520666200303130833 Haonan Zhang 1 , Zhengquan Gao 1 , Chunxiao Meng 1 , Xiangqian Li 2, 3 , Dayong Shi 2, 3
|
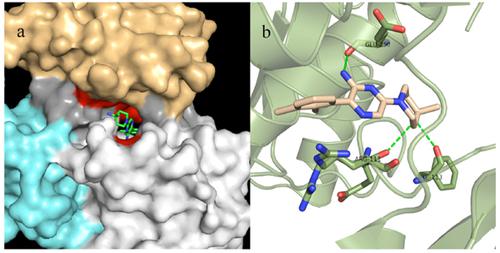
Protein tyrosine phosphatase 2 (SHP-2) has long been proposed as a cancer drug target. Several small-molecule compounds with different mechanisms of SHP-2 inhibition have been reported, but none are commercially available. Pool selectivity over protein tyrosine phosphatase 1 (SHP-1) and a lack of cellular activity have hindered the development of selective SHP-2 inhibitors. In this review, we describe the binding modes of existing inhibitors and SHP-2 binding sites, summarize the characteristics of the sites involved in selectivity, and identify the suitable groups for interaction with the binding sites.
中文翻译:

酪氨酸磷酸酶SHP-2中的抑制剂结合位点。
长期以来,人们一直提出蛋白质酪氨酸磷酸酶2(SHP-2)作为抗癌药物的靶标。已经报道了几种具有不同的SHP-2抑制机制的小分子化合物,但是没有一种可商购。对蛋白质酪氨酸磷酸酶1(SHP-1)的池选择性和缺乏细胞活性阻碍了选择性SHP-2抑制剂的发展。在这篇综述中,我们描述了现有抑制剂和SHP-2结合位点的结合方式,总结了选择性所涉及位点的特征,并确定了与结合位点相互作用的合适基团。


























 京公网安备 11010802027423号
京公网安备 11010802027423号