当前位置:
X-MOL 学术
›
Hum. Mutat.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Pathogenic PTPN11 variants involving the poly-glutamine Gln255 -Gln256 -Gln257 stretch highlight the relevance of helix B in SHP2's functional regulation.
Human Mutation ( IF 3.9 ) Pub Date : 2020-03-11 , DOI: 10.1002/humu.24007 Simone Martinelli 1 , Luca Pannone 1, 2 , Christina Lissewski 3 , Julia Brinkmann 3 , Elisabetta Flex 1 , Denny Schanze 3 , Paolo Calligari 4 , Massimiliano Anselmi 4 , Francesca Pantaleoni 2 , Viviana Claudia Canale 4 , Francesca Clementina Radio 2 , Adonis Ioannides 5, 6 , Nils Rahner 7 , Ina Schanze 3 , Dragana Josifova 6 , Gianfranco Bocchinfuso 4 , Mina Ryten 6 , Lorenzo Stella 4 , Marco Tartaglia 2 , Martin Zenker 3
Human Mutation ( IF 3.9 ) Pub Date : 2020-03-11 , DOI: 10.1002/humu.24007 Simone Martinelli 1 , Luca Pannone 1, 2 , Christina Lissewski 3 , Julia Brinkmann 3 , Elisabetta Flex 1 , Denny Schanze 3 , Paolo Calligari 4 , Massimiliano Anselmi 4 , Francesca Pantaleoni 2 , Viviana Claudia Canale 4 , Francesca Clementina Radio 2 , Adonis Ioannides 5, 6 , Nils Rahner 7 , Ina Schanze 3 , Dragana Josifova 6 , Gianfranco Bocchinfuso 4 , Mina Ryten 6 , Lorenzo Stella 4 , Marco Tartaglia 2 , Martin Zenker 3
Affiliation
|
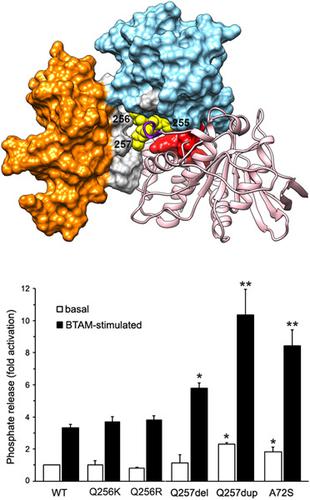
Germline PTPN11 mutations cause Noonan syndrome (NS), the most common disorder among RASopathies. PTPN11 encodes SHP2, a protein tyrosine-phosphatase controlling signaling through the RAS-MAPK and PI3K-AKT pathways. Generally, NS-causing PTPN11 mutations are missense changes destabilizing the inactive conformation of the protein or enhancing its binding to signaling partners. Here, we report on two PTPN11 variants resulting in the deletion or duplication of one of three adjacent glutamine residues (Gln255 -to-Gln257 ). While p.(Gln257dup) caused a typical NS phenotype in carriers of a first family, p.(Gln257del) had incomplete penetrance in a second family. Missense mutations involving Gln256 had previously been reported in NS. This poly-glutamine stretch is located on helix B of the PTP domain, a region involved in stabilizing SHP2 in its autoinhibited state. Molecular dynamics simulations predicted that changes affecting this motif perturb the SHP2's catalytically inactive conformation and/or substrate recognition. Biochemical data showed that duplication and deletion of Gln257 variably enhance SHP2's catalytic activity, while missense changes involving Gln256 affect substrate specificity. Expression of mutants in HEK293T cells documented their activating role on MAPK signaling, uncoupling catalytic activity and modulation of intracellular signaling. These findings further document the relevance of helix B in the regulation of SHP2's function.
中文翻译:

涉及聚谷氨酰胺 Gln255 -Gln256 -Gln257 延伸的致病性 PTPN11 变异突出了螺旋 B 在 SHP2 功能调节中的相关性。
生殖系 PTPN11 突变导致 Noonan 综合征 (NS),这是 RASopathies 中最常见的疾病。PTPN11 编码 SHP2,一种通过 RAS-MAPK 和 PI3K-AKT 通路控制信号的蛋白质酪氨酸-磷酸酶。通常,导致 NS 的 PTPN11 突变是错义变化,会破坏蛋白质的非活性构象或增强其与信号伙伴的结合。在这里,我们报告了两个 PTPN11 变体,导致三个相邻谷氨酰胺残基之一的缺失或重复(Gln255 -to-Gln257)。p.(Gln257dup) 在第一个家族的携带者中引起典型的 NS 表型,而 p.(Gln257del) 在第二个家族中具有不完全外显率。以前曾在 NS 中报道过涉及 Gln256 的错义突变。这种聚谷氨酰胺链位于 PTP 结构域的螺旋 B 上,参与将 SHP2 稳定在其自身抑制状态的区域。分子动力学模拟预测,影响该基序的变化会扰乱 SHP2 无催化活性的构象和/或底物识别。生化数据表明,Gln257 的重复和缺失不同程度地增强了 SHP2 的催化活性,而涉及 Gln256 的错义变化会影响底物特异性。HEK293T 细胞中突变体的表达证明了它们对 MAPK 信号传导、解偶联催化活性和细胞内信号传导的调节作用。这些发现进一步证明了螺旋 B 在 SHP2 功能调节中的相关性。生化数据表明,Gln257 的重复和缺失不同程度地增强了 SHP2 的催化活性,而涉及 Gln256 的错义变化会影响底物特异性。HEK293T 细胞中突变体的表达证明了它们对 MAPK 信号传导、解偶联催化活性和细胞内信号传导的调节作用。这些发现进一步证明了螺旋 B 在 SHP2 功能调节中的相关性。生化数据表明,Gln257 的重复和缺失不同程度地增强了 SHP2 的催化活性,而涉及 Gln256 的错义变化会影响底物特异性。HEK293T 细胞中突变体的表达证明了它们对 MAPK 信号传导、解偶联催化活性和细胞内信号传导的调节作用。这些发现进一步证明了螺旋 B 在 SHP2 功能调节中的相关性。
更新日期:2020-03-11
中文翻译:

涉及聚谷氨酰胺 Gln255 -Gln256 -Gln257 延伸的致病性 PTPN11 变异突出了螺旋 B 在 SHP2 功能调节中的相关性。
生殖系 PTPN11 突变导致 Noonan 综合征 (NS),这是 RASopathies 中最常见的疾病。PTPN11 编码 SHP2,一种通过 RAS-MAPK 和 PI3K-AKT 通路控制信号的蛋白质酪氨酸-磷酸酶。通常,导致 NS 的 PTPN11 突变是错义变化,会破坏蛋白质的非活性构象或增强其与信号伙伴的结合。在这里,我们报告了两个 PTPN11 变体,导致三个相邻谷氨酰胺残基之一的缺失或重复(Gln255 -to-Gln257)。p.(Gln257dup) 在第一个家族的携带者中引起典型的 NS 表型,而 p.(Gln257del) 在第二个家族中具有不完全外显率。以前曾在 NS 中报道过涉及 Gln256 的错义突变。这种聚谷氨酰胺链位于 PTP 结构域的螺旋 B 上,参与将 SHP2 稳定在其自身抑制状态的区域。分子动力学模拟预测,影响该基序的变化会扰乱 SHP2 无催化活性的构象和/或底物识别。生化数据表明,Gln257 的重复和缺失不同程度地增强了 SHP2 的催化活性,而涉及 Gln256 的错义变化会影响底物特异性。HEK293T 细胞中突变体的表达证明了它们对 MAPK 信号传导、解偶联催化活性和细胞内信号传导的调节作用。这些发现进一步证明了螺旋 B 在 SHP2 功能调节中的相关性。生化数据表明,Gln257 的重复和缺失不同程度地增强了 SHP2 的催化活性,而涉及 Gln256 的错义变化会影响底物特异性。HEK293T 细胞中突变体的表达证明了它们对 MAPK 信号传导、解偶联催化活性和细胞内信号传导的调节作用。这些发现进一步证明了螺旋 B 在 SHP2 功能调节中的相关性。生化数据表明,Gln257 的重复和缺失不同程度地增强了 SHP2 的催化活性,而涉及 Gln256 的错义变化会影响底物特异性。HEK293T 细胞中突变体的表达证明了它们对 MAPK 信号传导、解偶联催化活性和细胞内信号传导的调节作用。这些发现进一步证明了螺旋 B 在 SHP2 功能调节中的相关性。



























 京公网安备 11010802027423号
京公网安备 11010802027423号