当前位置:
X-MOL 学术
›
Genes Cells
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Sox2 gene regulation via the D1 enhancer in embryonic neural tube and neural crest by the combined action of SOX2 and ZIC2.
Genes to Cells ( IF 2.1 ) Pub Date : 2020-02-26 , DOI: 10.1111/gtc.12753 Hideaki Iida 1 , Yoko Furukawa 1 , Machiko Teramoto 1 , Hitomi Suzuki 2 , Tatsuya Takemoto 2 , Masanori Uchikawa 3 , Hisato Kondoh 1
Genes to Cells ( IF 2.1 ) Pub Date : 2020-02-26 , DOI: 10.1111/gtc.12753 Hideaki Iida 1 , Yoko Furukawa 1 , Machiko Teramoto 1 , Hitomi Suzuki 2 , Tatsuya Takemoto 2 , Masanori Uchikawa 3 , Hisato Kondoh 1
Affiliation
|
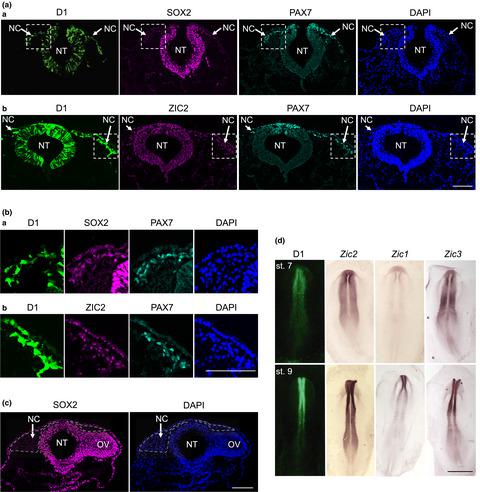
The transcription factor (TF) SOX2 regulates various stem cells and tissue progenitors via functional interactions with cell type-specific partner TFs that co-bind to enhancer sequences. Neural progenitors are the major embryonic tissues where SOX2 assumes central regulatory roles. In order to characterize the partner TFs of SOX2 in neural progenitors, we investigated the regulation of the D1 enhancer of the Sox2 gene, which is activated in the embryonic neural tube (NT) and neural crest (NC), using chicken embryo electroporation. We identified essential TF binding sites for a SOX, and two ZIC TFs in the activation of the D1 enhancer. By comparison of dorso-ventral and antero-posterior patterns of D1 enhancer activation, and the effect of mutations on the enhancer activation patterns with TF expression patterns, we determined SOX2 and ZIC2 as the major D1 enhancer-activating TFs. Binding of these TFs to the D1 enhancer sequence was confirmed by chromatin immunoprecipitation analysis. The combination of SOX2 and ZIC2 TFs activated the enhancer in both the NT and NC. These results indicate that SOX2 and ZIC2, which have been known to play major regulatory roles in neural progenitors, do functionally cooperate. In addition, the recently demonstrated SOX2 expression during the NC development is accounted for at least partly by the D1 enhancer activity. Deletion of the D1 enhancer sequence from the mouse genome, however, did not affect the mouse development, indicating functional redundancies of other enhancers.
中文翻译:

通过SOX2和ZIC2的联合作用,通过D1增强子调节胚胎神经管和神经neural中的Sox2基因。
转录因子(TF)SOX2通过与细胞类型特异性伴侣TF的功能相互作用来调节各种干细胞和组织祖细胞,后者与增强子序列共结合。神经祖细胞是SOX2承担主要调节作用的主要胚胎组织。为了表征SOX2在神经祖细胞中的伴侣TF,我们研究了Sox2基因D1增强子的调控,该基因在鸡胚神经管(NT)和神经neural(NC)中被激活,使用鸡胚电穿孔法。我们确定了SOX的基本TF结合位点,以及激活D1增强子的两个ZIC TF。通过比较D1增强子激活的背腹和前后模式,以及突变与TF表达模式对增强子激活模式的影响,我们确定SOX2和ZIC2是主要的D1增强剂激活TF。通过染色质免疫沉淀分析证实了这些TF与D1增强子序列的结合。SOX2和ZIC2 TF的组合激活了NT和NC中的增强子。这些结果表明,已知在神经祖细胞中起主要调节作用的SOX2和ZIC2在功能上相互配合。另外,在NC发展过程中最近证明的SOX2表达至少部分地由D1增强子活性引起。但是,从小鼠基因组中删除D1增强子序列并不会影响小鼠的发育,这表明其他增强子在功能上存在冗余。SOX2和ZIC2 TF的组合激活了NT和NC中的增强子。这些结果表明,已知在神经祖细胞中起主要调节作用的SOX2和ZIC2在功能上相互配合。另外,在NC发展过程中最近证明的SOX2表达至少部分地由D1增强子活性引起。但是,从小鼠基因组中删除D1增强子序列并不会影响小鼠的发育,这表明其他增强子在功能上存在冗余。SOX2和ZIC2 TF的组合激活了NT和NC中的增强子。这些结果表明,已知在神经祖细胞中起主要调控作用的SOX2和ZIC2在功能上相互配合。另外,在NC发展过程中最近证明的SOX2表达至少部分地由D1增强子活性引起。但是,从小鼠基因组中删除D1增强子序列并不会影响小鼠的发育,这表明其他增强子在功能上存在冗余。在NC发育过程中最近证实的SOX2表达至少部分地由D1增强子活性引起。但是,从小鼠基因组中删除D1增强子序列并不会影响小鼠的发育,这表明其他增强子在功能上存在冗余。在NC发育过程中最近证实的SOX2表达至少部分地由D1增强子活性引起。但是,从小鼠基因组中删除D1增强子序列并不会影响小鼠的发育,这表明其他增强子在功能上存在冗余。
更新日期:2020-02-26
中文翻译:

通过SOX2和ZIC2的联合作用,通过D1增强子调节胚胎神经管和神经neural中的Sox2基因。
转录因子(TF)SOX2通过与细胞类型特异性伴侣TF的功能相互作用来调节各种干细胞和组织祖细胞,后者与增强子序列共结合。神经祖细胞是SOX2承担主要调节作用的主要胚胎组织。为了表征SOX2在神经祖细胞中的伴侣TF,我们研究了Sox2基因D1增强子的调控,该基因在鸡胚神经管(NT)和神经neural(NC)中被激活,使用鸡胚电穿孔法。我们确定了SOX的基本TF结合位点,以及激活D1增强子的两个ZIC TF。通过比较D1增强子激活的背腹和前后模式,以及突变与TF表达模式对增强子激活模式的影响,我们确定SOX2和ZIC2是主要的D1增强剂激活TF。通过染色质免疫沉淀分析证实了这些TF与D1增强子序列的结合。SOX2和ZIC2 TF的组合激活了NT和NC中的增强子。这些结果表明,已知在神经祖细胞中起主要调节作用的SOX2和ZIC2在功能上相互配合。另外,在NC发展过程中最近证明的SOX2表达至少部分地由D1增强子活性引起。但是,从小鼠基因组中删除D1增强子序列并不会影响小鼠的发育,这表明其他增强子在功能上存在冗余。SOX2和ZIC2 TF的组合激活了NT和NC中的增强子。这些结果表明,已知在神经祖细胞中起主要调节作用的SOX2和ZIC2在功能上相互配合。另外,在NC发展过程中最近证明的SOX2表达至少部分地由D1增强子活性引起。但是,从小鼠基因组中删除D1增强子序列并不会影响小鼠的发育,这表明其他增强子在功能上存在冗余。SOX2和ZIC2 TF的组合激活了NT和NC中的增强子。这些结果表明,已知在神经祖细胞中起主要调控作用的SOX2和ZIC2在功能上相互配合。另外,在NC发展过程中最近证明的SOX2表达至少部分地由D1增强子活性引起。但是,从小鼠基因组中删除D1增强子序列并不会影响小鼠的发育,这表明其他增强子在功能上存在冗余。在NC发育过程中最近证实的SOX2表达至少部分地由D1增强子活性引起。但是,从小鼠基因组中删除D1增强子序列并不会影响小鼠的发育,这表明其他增强子在功能上存在冗余。在NC发育过程中最近证实的SOX2表达至少部分地由D1增强子活性引起。但是,从小鼠基因组中删除D1增强子序列并不会影响小鼠的发育,这表明其他增强子在功能上存在冗余。


























 京公网安备 11010802027423号
京公网安备 11010802027423号