Current Organic Synthesis ( IF 1.8 ) Pub Date : 2020-05-01 , DOI: 10.2174/1570179417666200218092047 Magda H Abdellattif 1 , Ola Abu Ali 2 , Mohamed M H Arief 3 , Mostafa A Hussien 4, 5
|
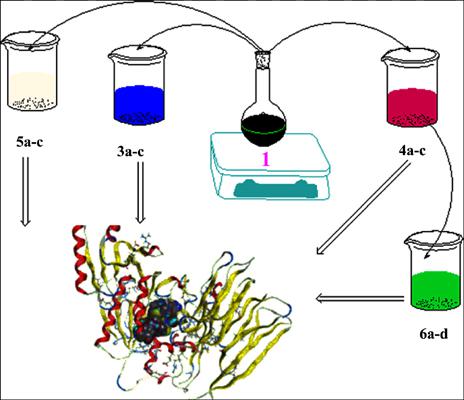
Background: The synthesis of a novel series of oxadiazine-4-thione biological molecules was executed through the incorporation of the ortho-, meta-, and para-benzoyl isocyanates to the tetrabromophthalimide nucleus.
Objectives: A one-pot multicomponent methodology in a solvent-free microwave irradiation environment was employed to afford this series of oxadiazine-4-thione, deriving a comparison with the conventional method. Subsequently, the yielded derivatives were subjected to further biological assessment.
Materials and Methods: The acquired results denoted that the one-pot procedure, which delivered products in a 2-4 min. interval, was more efficient in evaluation against the classical method, which consumed a 1-2:30 hr. interval.
Results: The application of the antibacterial analyses was subjected to all the compounds, resulting in molecules 6a and 6c demonstrating the highest activity regarding Aspergillus Favus; molecules 5b and 5c exhibiting an equivalent level of activity towards E-coli and Fusarium Moniliform; and molecules 4b, 4c, 5b, and 5c presenting an identical level of activity to the aforementioned derivatives involving Staphylococcus.
Concluison: Molecular modeling studies by the MOE, the preceding antibacterial behavior was conducted to advocate the newly prepared compounds. Moreover, the spectroscopic approaches were exploited to verify and establish the structures and mechanisms of the synthesized derivatives’ reactions.
中文翻译:

一锅法合成新颖的恶二嗪-4-硫酮衍生物及其抗菌活性和分子模型研究。
背景:通过将邻,间和对苯甲酰基异氰酸酯掺入四溴邻苯二甲酰亚胺核中,完成了一系列新的恶二嗪-4-硫酮生物分子的合成。
目的:在无溶剂微波辐射环境中采用一锅法多组分方法制得该系列恶二嗪-4-硫酮,与常规方法进行了比较。随后,将所得的衍生物进行进一步的生物学评估。
材料和方法:获得的结果表示一锅法,该方法在2-4分钟内交付了产品。间隔时间比传统方法的评估效率更高,后者需要1-2:30 hr。间隔。
结果:所有化合物均经过了抗菌分析,结果分子6a和6c表现出关于曲霉曲霉的最高活性。分子5b和5c对大肠杆菌和镰孢镰刀菌具有同等水平的活性;分子4b,4c,5b和5c具有与上述涉及葡萄球菌的衍生物相同水平的活性。
结论:由教育部进行分子模型研究,进行了先前的抗菌行为以提倡新制备的化合物。此外,利用光谱学方法验证并建立了合成衍生物反应的结构和机理。



























 京公网安备 11010802027423号
京公网安备 11010802027423号