当前位置:
X-MOL 学术
›
J. Exp. Zool. B Mol. Dev. Evol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Evolution of epimorphosis in mammals
Journal of Experimental Zoology-B: Molecular and Developmental Evolution ( IF 2.2 ) Pub Date : 2020-01-17 , DOI: 10.1002/jez.b.22925 Ken Muneoka 1 , Lindsay A Dawson 1
Journal of Experimental Zoology-B: Molecular and Developmental Evolution ( IF 2.2 ) Pub Date : 2020-01-17 , DOI: 10.1002/jez.b.22925 Ken Muneoka 1 , Lindsay A Dawson 1
Affiliation
|
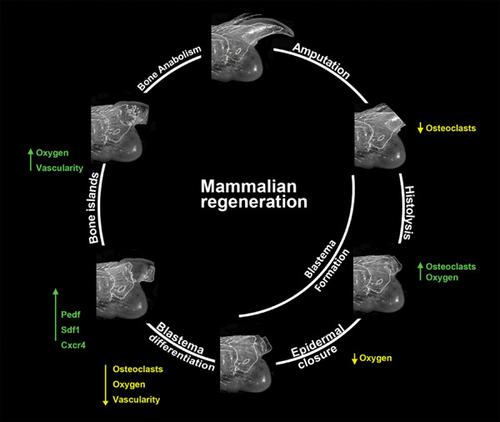
Mammalian epimorphic regeneration is rare and digit tip regeneration in mice is the best‐studied model for a multi‐tissue regenerative event that involves blastema formation. Digit tip regeneration parallels human fingertip regeneration, thus understanding the details of this response can provide insight into developing strategies to expand the potential of human regeneration. Following amputation, the digit stump undergoes a strong histolytic response involving osteoclast‐mediated bone degradation that is spatially and temporally linked to the expansion of blastema osteoprogenitor cells. Blastemal differentiation occurs via direct intramembranous ossification. Although robust, digit regeneration is imperfect: The amputated cortical bone is replaced with woven bone and there is excessive bone regeneration restricted to the dorsal–ventral axis. Ontogenetic and phylogenetic analysis of digit regeneration in amphibians and mammals raise the possibility that mammalian blastema is a product of convergent evolution and we hypothesize that digit tip regeneration evolved from a nonregenerative precondition. A model is proposed in which the mammalian blastema evolved in part from an adaptation of two bone repair strategies (the bone remodeling cycle and fracture healing) both of which are conserved across tetrapod vertebrates. The view that epimorphic regeneration evolved in mammals from a nonregenerative precondition is supported by recent studies demonstrating that complex regenerative responses can be induced from a number of different nonregenerative amputation wounds by specific modification of the healing response.
中文翻译:

哺乳动物表态的进化
哺乳动物表形再生是罕见的,小鼠指尖再生是涉及胚泡形成的多组织再生事件的最佳研究模型。指尖再生与人类指尖再生平行,因此了解这种反应的细节可以深入了解开发策略以扩大人类再生的潜力。截肢后,手指残端经历强烈的组织溶解反应,涉及破骨细胞介导的骨降解,这在空间和时间上与胚细胞骨祖细胞的扩增有关。通过直接的膜内骨化发生母细胞分化。虽然强壮,但手指再生并不完美:截肢的皮质骨被编织骨取代,过度的骨再生仅限于背腹轴。两栖动物和哺乳动物手指再生的个体发育和系统发育分析提出了哺乳动物胚泡是趋同进化的产物的可能性,我们假设指尖再生是从非再生先决条件进化而来的。提出了一个模型,其中哺乳动物的胚泡部分进化自两种骨修复策略(骨重塑周期和骨折愈合)的适应,这两种策略在四足脊椎动物中都是保守的。最近的研究支持了哺乳动物从非再生先决条件进化而来的表观再生的观点,这些研究表明,通过对愈合反应的特定修改,可以从许多不同的非再生截肢伤口中诱导复杂的再生反应。
更新日期:2020-01-17
中文翻译:

哺乳动物表态的进化
哺乳动物表形再生是罕见的,小鼠指尖再生是涉及胚泡形成的多组织再生事件的最佳研究模型。指尖再生与人类指尖再生平行,因此了解这种反应的细节可以深入了解开发策略以扩大人类再生的潜力。截肢后,手指残端经历强烈的组织溶解反应,涉及破骨细胞介导的骨降解,这在空间和时间上与胚细胞骨祖细胞的扩增有关。通过直接的膜内骨化发生母细胞分化。虽然强壮,但手指再生并不完美:截肢的皮质骨被编织骨取代,过度的骨再生仅限于背腹轴。两栖动物和哺乳动物手指再生的个体发育和系统发育分析提出了哺乳动物胚泡是趋同进化的产物的可能性,我们假设指尖再生是从非再生先决条件进化而来的。提出了一个模型,其中哺乳动物的胚泡部分进化自两种骨修复策略(骨重塑周期和骨折愈合)的适应,这两种策略在四足脊椎动物中都是保守的。最近的研究支持了哺乳动物从非再生先决条件进化而来的表观再生的观点,这些研究表明,通过对愈合反应的特定修改,可以从许多不同的非再生截肢伤口中诱导复杂的再生反应。


























 京公网安备 11010802027423号
京公网安备 11010802027423号