当前位置:
X-MOL 学术
›
Chem. Asian J.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Rational Synthesis of 5,5,5-Tricyclic Fused Thia-heptaphyrin (1.1.1.1.1.1.0) From a Helical Oligopyrrin Hybrid.
Chemistry - An Asian Journal ( IF 4.1 ) Pub Date : 2020-03-13 , DOI: 10.1002/asia.202000100 Yating Fu 1 , Xiujun Liu 1 , Chengjie Li 1 , Zhangfa Tong 2 , Glib Baryshnikov 3 , Hans Ågren 3 , Qizhao Li 1 , Yongshu Xie 1, 2
Chemistry - An Asian Journal ( IF 4.1 ) Pub Date : 2020-03-13 , DOI: 10.1002/asia.202000100 Yating Fu 1 , Xiujun Liu 1 , Chengjie Li 1 , Zhangfa Tong 2 , Glib Baryshnikov 3 , Hans Ågren 3 , Qizhao Li 1 , Yongshu Xie 1, 2
Affiliation
|
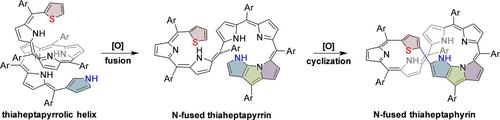
Oxidation of a thiophene-hexapyrrane hybrid S-P6 afforded a stable conjugated open-chain thiaheptapyrrolic helix 1 with the terminal thiophene and confused pyrrole units lying at a long distance that is adverse for further cyclization. Chelation of 1 with copper(II) ion afforded 1-Cu, which exhibits more distant terminal units. Interestingly, further oxidation of 1 triggered an intramolecular C-N fusion reaction to afford a unique 5,5,5-tricyclic fused linear thiaheptapyrrin 2, with the two terminals positioned in proximity, which favors the oxidative ring-closure reaction to give a unique 5,5,5-tricyclic fused thiaheptaphyrin (1.1.1.1.1.1.0) 3 under air. The inner-fusion strategy for positioning the reactive sites in proximity to promote oxidative cyclization offers a new approach for constructing large porphyrinoids through conjugated oligopyrrins without the assistance of metal ions.
中文翻译:

从螺旋寡吡喃杂化物中合理合成5,5,5-三环稠合硫杂庚酸(1.1.1.1.1.1.0)。
噻吩-六吡喃杂化物S-P6的氧化可得到稳定的共轭开环噻庚并吡咯螺旋1,末端噻吩和吡咯单元的距离较长,这不利于进一步环化。1与铜(II)离子的螯合得到1-Cu,它具有更远的末端单元。有趣的是,进一步氧化1会触发分子内CN融合反应,从而产生独特的5,5,5-三环稠合线性硫庚啶2,两个末端均位于附近,这有利于氧化闭环反应产生独特的5,空气中的5,5-三环稠合硫庚七素(1.1.1.1.1.1.0)3
更新日期:2020-04-22
中文翻译:

从螺旋寡吡喃杂化物中合理合成5,5,5-三环稠合硫杂庚酸(1.1.1.1.1.1.0)。
噻吩-六吡喃杂化物S-P6的氧化可得到稳定的共轭开环噻庚并吡咯螺旋1,末端噻吩和吡咯单元的距离较长,这不利于进一步环化。1与铜(II)离子的螯合得到1-Cu,它具有更远的末端单元。有趣的是,进一步氧化1会触发分子内CN融合反应,从而产生独特的5,5,5-三环稠合线性硫庚啶2,两个末端均位于附近,这有利于氧化闭环反应产生独特的5,空气中的5,5-三环稠合硫庚七素(1.1.1.1.1.1.0)3


























 京公网安备 11010802027423号
京公网安备 11010802027423号