当前位置:
X-MOL 学术
›
STEM CELLS
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Hes1 deficiency causes hematopoietic stem cell exhaustion
STEM CELLS ( IF 5.2 ) Pub Date : 2020-03-16 , DOI: 10.1002/stem.3169 Zhilin Ma 1, 2 , Jian Xu 1, 2 , Limei Wu 1 , Junjie Wang 1 , Qiqi Lin 1, 2 , Fabliha A Chowdhury 1 , Md Habibul H Mazumder 1 , Gangqing Hu 3, 4 , Xue Li 1, 2 , Wei Du 1, 5
STEM CELLS ( IF 5.2 ) Pub Date : 2020-03-16 , DOI: 10.1002/stem.3169 Zhilin Ma 1, 2 , Jian Xu 1, 2 , Limei Wu 1 , Junjie Wang 1 , Qiqi Lin 1, 2 , Fabliha A Chowdhury 1 , Md Habibul H Mazumder 1 , Gangqing Hu 3, 4 , Xue Li 1, 2 , Wei Du 1, 5
Affiliation
|
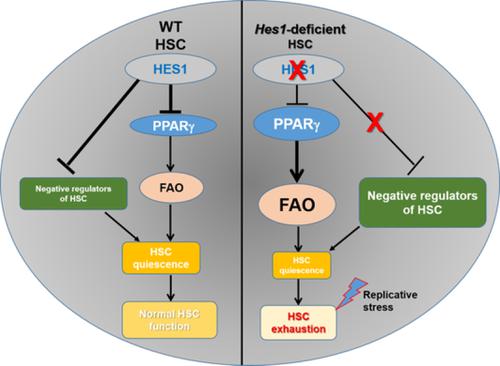
The transcriptional repressor Hairy Enhancer of Split 1 (HES1) plays an essential role in the development of many organs by promoting the maintenance of stem/progenitor cells, controlling the reversibility of cellular quiescence, and regulating both cell fate decisions. Deletion of Hes1 in mice results in severe defects in multiple organs and is lethal in late embryogenesis. Here we have investigated the role of HES1 in hematopoiesis using a hematopoietic lineage‐specific Hes1 knockout mouse model. We found that while Hes1 is dispensable for steady‐state hematopoiesis, Hes1‐deficient hematopoietic stem cells (HSCs) undergo exhaustion under replicative stress. Loss of Hes1 upregulates the expression of genes involved in PPARγ signaling and fatty acid metabolism pathways, and augments fatty acid oxidation (FAO) in Hes1 f/fVav1Cre HSCs and progenitors. Functionally, PPARγ targeting or FAO inhibition ameliorates the repopulating defects of Hes1 f/fVav1Cre HSCs through improving quiescence in HSCs. Lastly, transcriptome analysis reveals that disruption of Hes1 in hematopoietic lineage alters expression of genes critical to HSC function, PPARγ signaling, and fatty acid metabolism. Together, our findings identify a novel role of HES1 in regulating stress hematopoiesis and provide mechanistic insight into the function of HES1 in HSC maintenance.
中文翻译:

Hes1缺乏导致造血干细胞耗竭
Split 1 (HES1) 的转录抑制因子毛状增强子通过促进干/祖细胞的维持、控制细胞静止的可逆性和调节两种细胞命运决定,在许多器官的发育中发挥重要作用。在小鼠中缺失 Hes1 会导致多个器官的严重缺陷,并且在胚胎发生后期是致命的。在这里,我们使用造血谱系特异性 Hes1 敲除小鼠模型研究了 HES1 在造血中的作用。我们发现,虽然 Hes1 对稳态造血是可有可无的,但 Hes1 缺陷的造血干细胞 (HSC) 在复制压力下会耗竭。Hes1 的缺失上调了参与 PPARγ 信号传导和脂肪酸代谢途径的基因的表达,并增强 Hes1 f/fVav1Cre HSC 和祖细胞中的脂肪酸氧化 (FAO)。在功能上,PPARγ 靶向或 FAO 抑制通过改善 HSC 的静止状态来改善 Hes1 f/fVav1Cre HSC 的再增殖缺陷。最后,转录组分析表明,造血谱系中 Hes1 的破坏会改变对 HSC 功能、PPARγ 信号传导和脂肪酸代谢至关重要的基因的表达。总之,我们的研究结果确定了 HES1 在调节应激造血中的新作用,并提供了对 HES1 在 HSC 维持中功能的机制洞察。转录组分析表明,造血谱系中 Hes1 的破坏会改变对 HSC 功能、PPARγ 信号传导和脂肪酸代谢至关重要的基因的表达。总之,我们的研究结果确定了 HES1 在调节应激造血中的新作用,并提供了对 HES1 在 HSC 维持中功能的机制洞察。转录组分析表明,造血谱系中 Hes1 的破坏会改变对 HSC 功能、PPARγ 信号传导和脂肪酸代谢至关重要的基因的表达。总之,我们的研究结果确定了 HES1 在调节应激造血中的新作用,并提供了对 HES1 在 HSC 维持中功能的机制洞察。
更新日期:2020-03-16
中文翻译:

Hes1缺乏导致造血干细胞耗竭
Split 1 (HES1) 的转录抑制因子毛状增强子通过促进干/祖细胞的维持、控制细胞静止的可逆性和调节两种细胞命运决定,在许多器官的发育中发挥重要作用。在小鼠中缺失 Hes1 会导致多个器官的严重缺陷,并且在胚胎发生后期是致命的。在这里,我们使用造血谱系特异性 Hes1 敲除小鼠模型研究了 HES1 在造血中的作用。我们发现,虽然 Hes1 对稳态造血是可有可无的,但 Hes1 缺陷的造血干细胞 (HSC) 在复制压力下会耗竭。Hes1 的缺失上调了参与 PPARγ 信号传导和脂肪酸代谢途径的基因的表达,并增强 Hes1 f/fVav1Cre HSC 和祖细胞中的脂肪酸氧化 (FAO)。在功能上,PPARγ 靶向或 FAO 抑制通过改善 HSC 的静止状态来改善 Hes1 f/fVav1Cre HSC 的再增殖缺陷。最后,转录组分析表明,造血谱系中 Hes1 的破坏会改变对 HSC 功能、PPARγ 信号传导和脂肪酸代谢至关重要的基因的表达。总之,我们的研究结果确定了 HES1 在调节应激造血中的新作用,并提供了对 HES1 在 HSC 维持中功能的机制洞察。转录组分析表明,造血谱系中 Hes1 的破坏会改变对 HSC 功能、PPARγ 信号传导和脂肪酸代谢至关重要的基因的表达。总之,我们的研究结果确定了 HES1 在调节应激造血中的新作用,并提供了对 HES1 在 HSC 维持中功能的机制洞察。转录组分析表明,造血谱系中 Hes1 的破坏会改变对 HSC 功能、PPARγ 信号传导和脂肪酸代谢至关重要的基因的表达。总之,我们的研究结果确定了 HES1 在调节应激造血中的新作用,并提供了对 HES1 在 HSC 维持中功能的机制洞察。


























 京公网安备 11010802027423号
京公网安备 11010802027423号