当前位置:
X-MOL 学术
›
ChemPhysChem
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
How Ligand Binding Affects the Dynamical Transition Temperature in Proteins.
ChemPhysChem ( IF 2.9 ) Pub Date : 2020-04-01 , DOI: 10.1002/cphc.201901221 Alexander Krah 1, 2 , Roland G Huber 2 , Peter J Bond 2, 3
ChemPhysChem ( IF 2.9 ) Pub Date : 2020-04-01 , DOI: 10.1002/cphc.201901221 Alexander Krah 1, 2 , Roland G Huber 2 , Peter J Bond 2, 3
Affiliation
|
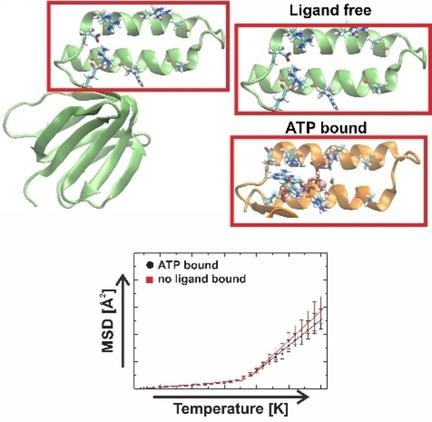
The biochemical functions of proteins are activated at the protein glass transition temperature, which has been proposed to be dependent upon protein‐water interactions. However, at the molecular level it is unclear how ligand binding to well‐defined binding sites can influence this transition temperature. We thus report molecular dynamics (MD) simulations of the ϵ subunit from thermophilic Bacillus PS3 in the ATP‐free and ligand‐bound states over a range of temperatures from 20 to 300 K, to study the influence of ligand association upon the transition temperature. We also measure the protein mean square displacement (MSD) in each state, which is well established as a means to quantify this dynamical temperature dependence. We find that the transition temperature is largely unaffected by ligand association, but the MSD beyond the transition temperature increases more rapidly in the ATP‐free state. Our data suggests that ligands can effectively “shield” a binding site from solvent, and hence stabilize protein domains with increasing temperature.
中文翻译:

配体结合如何影响蛋白质的动态转变温度。
蛋白质的生化功能在蛋白质玻璃化转变温度下被激活,这被认为取决于蛋白质与水的相互作用。但是,在分子水平上,尚不清楚配体与明确定义的结合位点的结合如何影响该转变温度。因此,我们报告了嗜热芽孢杆菌the亚基的分子动力学(MD)模拟PS3在20至300 K的温度范围内处于无ATP和配体结合状态,以研究配体缔合对转变温度的影响。我们还测量了每种状态下的蛋白质均方位移(MSD),这是量化这种动态温度依赖性的一种手段。我们发现,转变温度在很大程度上不受配体缔合的影响,但是在无ATP的状态下,超过转变温度的MSD增长更快。我们的数据表明,配体可以有效地“屏蔽”溶剂中的结合位点,从而随着温度的升高稳定蛋白质结构域。
更新日期:2020-04-01
中文翻译:

配体结合如何影响蛋白质的动态转变温度。
蛋白质的生化功能在蛋白质玻璃化转变温度下被激活,这被认为取决于蛋白质与水的相互作用。但是,在分子水平上,尚不清楚配体与明确定义的结合位点的结合如何影响该转变温度。因此,我们报告了嗜热芽孢杆菌the亚基的分子动力学(MD)模拟PS3在20至300 K的温度范围内处于无ATP和配体结合状态,以研究配体缔合对转变温度的影响。我们还测量了每种状态下的蛋白质均方位移(MSD),这是量化这种动态温度依赖性的一种手段。我们发现,转变温度在很大程度上不受配体缔合的影响,但是在无ATP的状态下,超过转变温度的MSD增长更快。我们的数据表明,配体可以有效地“屏蔽”溶剂中的结合位点,从而随着温度的升高稳定蛋白质结构域。


























 京公网安备 11010802027423号
京公网安备 11010802027423号