当前位置:
X-MOL 学术
›
WIREs Comput. Mol. Sci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
P2Y1‐like nucleotide receptors—Structures, molecular modeling, mutagenesis, and oligomerization
Wiley Interdisciplinary Reviews: Computational Molecular Science ( IF 11.4 ) Pub Date : 2020-03-03 , DOI: 10.1002/wcms.1464 Alexander Neumann 1, 2 , Christa E. Müller 1, 2 , Vigneshwaran Namasivayam 1
Wiley Interdisciplinary Reviews: Computational Molecular Science ( IF 11.4 ) Pub Date : 2020-03-03 , DOI: 10.1002/wcms.1464 Alexander Neumann 1, 2 , Christa E. Müller 1, 2 , Vigneshwaran Namasivayam 1
Affiliation
|
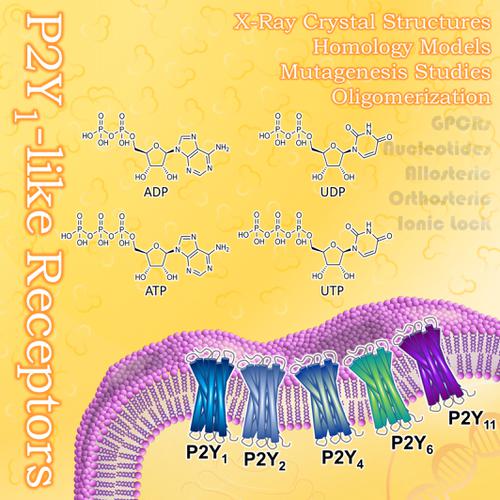
The P2Y receptors (P2YRs) are G protein‐coupled receptors (GPCRs) consisting of eight members, subdivided into two groups, P2Y1‐ and P2Y12‐like receptor subtypes. They are activated by extracellular nucleotides and represent current (P2Y2, P2Y12) or potential future drug targets. The chemical nature of the highly polar endogenous agonists represents a challenge in the discovery and design of potent and bioavailable compounds. A number of mutants and several homology models of P2YR subtypes have been created and updated on the basis of the recently published X‐ray crystal structures of the human P2Y1 and P2Y12Rs. The models were used for prediction of the binding sites of agonists and antagonists, and mutants were constructed for confirmation. Pharmacological data on mutants published for the P2Y1‐like receptors (P2Y1, P2Y2, P2Y4, P2Y6, and P2Y11R) were evaluated to analyze the role of specific amino acids and that of corresponding amino acid residues in related P2Y receptor subtypes. In several P2YR subtypes, an ionic lock between extracellular loop 2 and transmembrane region VII was postulated to be essential for agonist‐induced receptor activation. Mutagenesis and homology modeling data suggest that the nucleotide antagonist (1′R ,2′S ,4′S ,5′S )‐4‐(2‐iodo‐6‐methylaminopurin‐9‐yl)‐1‐[(phosphato)methyl]‐2‐(phosphato)bicyclo[3.1.0]hexane (MRS2500), which was co‐crystallized with the human P2Y1R, binds differently from agonistic nucleotides to a site partly overlapping with that of orthosteric agonists. Hetero‐oligomerization of P2YRs with other P2YR subtypes or other GPCRs may allosterically modulate receptor‐ligand interactions and/or G protein coupling. The collected information will contribute to the understanding of the architecture of P2Y1‐like nucleotide receptors and will consequently be useful for the design of novel agonists and antagonists.
中文翻译:

P2Y1样核苷酸受体—结构,分子建模,诱变和寡聚
P2Y受体(P2YRs)是G蛋白偶联受体(GPCR),由8个成员组成,分为P2Y 1和P2Y 12样受体亚型两组。它们被细胞外核苷酸激活,代表当前(P2Y 2,P2Y 12)或潜在的未来药物靶标。高极性内源性激动剂的化学性质对有效和生物利用性化合物的发现和设计提出了挑战。基于最近发布的人类P2Y 1和P2Y 12的X射线晶体结构,已经创建和更新了许多P2YR亚型的突变体和几种同源性模型。卢比 该模型用于预测激动剂和拮抗剂的结合位点,并构建突变体用于确认。评估了针对P2Y 1样受体(P2Y 1,P2Y 2,P2Y 4,P2Y 6和P2Y 11 R)发布的突变体的药理数据,以分析特定氨基酸的作用以及相关P2Y中相应氨基酸残基的作用受体亚型。在几种P2YR亚型中,推测细胞外环2与跨膜区VII之间的离子锁对激动剂诱导的受体激活必不可少。诱变和同源性建模数据表明核苷酸拮抗剂(1'R,2 'S,4 ′S,5 ′S)‐4‐(2-碘‐6‐甲基氨基嘌呤‐9‐yl)‐1 ‐ [((磷酸基)甲基] ‐2‐(磷酸基)双环[3.1.0]己烷(MRS2500)与人P2Y 1 R共结晶,与激动核苷酸的结合不同,其位点与正构激动剂部分重叠。P2YR与其他P2YR亚型或其他GPCR的异源寡聚化可能会变构地调节受体-配体相互作用和/或G蛋白偶联。所收集的信息将有助于理解P2Y 1样核苷酸受体的结构,因此对设计新型激动剂和拮抗剂很有用。
更新日期:2020-03-03
中文翻译:

P2Y1样核苷酸受体—结构,分子建模,诱变和寡聚
P2Y受体(P2YRs)是G蛋白偶联受体(GPCR),由8个成员组成,分为P2Y 1和P2Y 12样受体亚型两组。它们被细胞外核苷酸激活,代表当前(P2Y 2,P2Y 12)或潜在的未来药物靶标。高极性内源性激动剂的化学性质对有效和生物利用性化合物的发现和设计提出了挑战。基于最近发布的人类P2Y 1和P2Y 12的X射线晶体结构,已经创建和更新了许多P2YR亚型的突变体和几种同源性模型。卢比 该模型用于预测激动剂和拮抗剂的结合位点,并构建突变体用于确认。评估了针对P2Y 1样受体(P2Y 1,P2Y 2,P2Y 4,P2Y 6和P2Y 11 R)发布的突变体的药理数据,以分析特定氨基酸的作用以及相关P2Y中相应氨基酸残基的作用受体亚型。在几种P2YR亚型中,推测细胞外环2与跨膜区VII之间的离子锁对激动剂诱导的受体激活必不可少。诱变和同源性建模数据表明核苷酸拮抗剂(1'R,2 'S,4 ′S,5 ′S)‐4‐(2-碘‐6‐甲基氨基嘌呤‐9‐yl)‐1 ‐ [((磷酸基)甲基] ‐2‐(磷酸基)双环[3.1.0]己烷(MRS2500)与人P2Y 1 R共结晶,与激动核苷酸的结合不同,其位点与正构激动剂部分重叠。P2YR与其他P2YR亚型或其他GPCR的异源寡聚化可能会变构地调节受体-配体相互作用和/或G蛋白偶联。所收集的信息将有助于理解P2Y 1样核苷酸受体的结构,因此对设计新型激动剂和拮抗剂很有用。



























 京公网安备 11010802027423号
京公网安备 11010802027423号