Communications Chemistry ( IF 5.9 ) Pub Date : 2020-03-04 , DOI: 10.1038/s42004-020-0273-6 Jingshuo Gao 1 , Qin Xiong 1 , Xueting Wu 1 , Jiajie Deng 1 , Xiaocui Zhang 1 , Xiaohu Zhao 1 , Pengchi Deng 2 , Zhipeng Yu 1
|
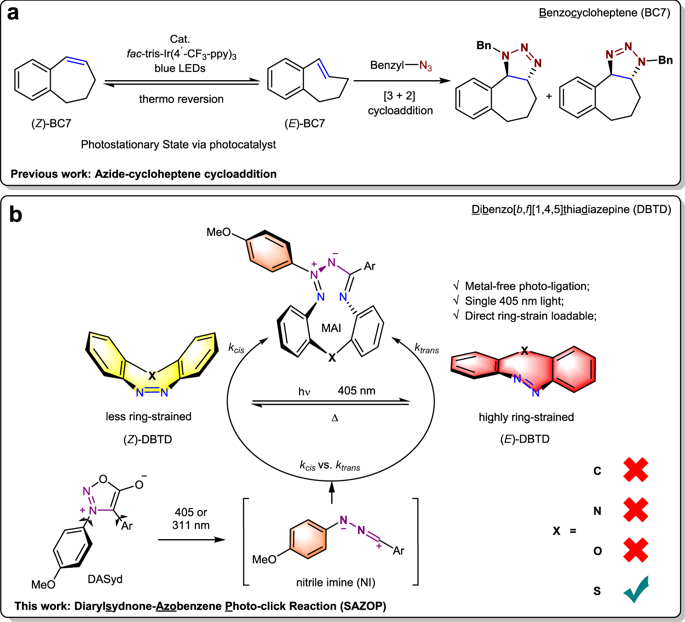
Ultra-fast and selective covalent-bond forming reactions with spatiotemporal controllability are foundational for developing a bioorthogonal approach with high manipulability. However, it is challenging to exploit a reporter functional group to achieve these requirements simultaneously. Here, 11H-Dibenzo[c,f][1,2]diazepine and a set of heterocyclic analogues are investigated for both their photo-switching natures and their ability to serve as dipolarophiles in photo-click reactions with diarylsydnone. Sulfur-containing dibenzothiadiazepine (DBTD) is discovered to be an excellent chemical reporter in cycloaddition with visible-light excitation for in-situ ring-strain loading via its (Z) → (E) photo-isomerization. The bioorthogonal utility of the DBTD tag in spatiotemporally controlled ligation for protein modifications on live cells is also demonstrated.
中文翻译:

通过二芳基苯并二苯并 [ b , f ] [1,4,5] 噻二氮卓照片点击反应进行可见光加速生物正交连接的直接环应变加载
具有时空可控性的超快速和选择性共价键形成反应是开发具有高可操作性的生物正交方法的基础。然而,利用报告功能组同时满足这些要求具有挑战性。在这里,研究了 11 H-二苯并 [ c , f ][1,2] 二氮杂卓和一组杂环类似物的光开关性质以及它们在与二芳基酮的光点击反应中作为亲偶极试剂的能力。含硫二苯并噻二氮卓 (DBTD) 被发现是一种出色的环加成化学报告剂,可见光激发通过其 ( Z ) → ( E) 光异构化。还证明了 DBTD 标签在时空控制的活细胞蛋白质修饰连接中的生物正交效用。



























 京公网安备 11010802027423号
京公网安备 11010802027423号