Microporous and Mesoporous Materials ( IF 5.2 ) Pub Date : 2020-03-03 , DOI: 10.1016/j.micromeso.2020.110134 Daniele Marciano , Boris Smolkin , Hadar Rotter , Ishay Columbus , Shani Pittel , Yakir Ophir , Alexander Pevzner
|
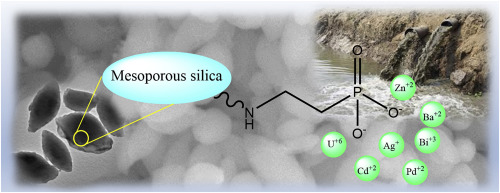
Phosphonate and/or bisphosphonate-functionalized mesoporous silica (MS) sorbents were developed and studied using 31P-MAS-NMR, SEM-EDS, TEM, BET, and batch sorption experiments before and/or after metal ion scavenging (Bi+3, Ba+2, Cd+2, U+6, Ag+, Pd+2 and Zn+2). The amount of phosphorus inside the sorbents was ascertained by SEM-EDS, and the phosphonate vs bisphosphonate content derived from NMR. The organic matter loading was between 15 and 38 %w/w and inversely correlated with BET. Pore size (4.9-6.6 nm) and surface areas (219-473 m2/g) were derived from nitrogen sorption isotherms. The NMR signals of the phosphonates are shifted downfield by the metal cations while the bisphosphonate signals are shifted upfield for urea-derived sorbents and downfield for the amino sorbent. The sorbents are composed of prolate particles of micrometric dimensions. The prolate particles of the urea-derived sorbents are stable following the adsorption of metal cations, while they are disrupted in the case of the amino sorbent, due to the repulsion between negative charges at basic pH. Only metal cations with relatively large ionic diameters were adsorbed revealing the need for multi-point interactions inside the sorbent, which is not possible for smaller cations. For all the sorbents, the sorption data of uranyl fit the Freundlich isotherm model, which predicts a heterogeneous surface. The K and Kd values derived from this model show good sorption capabilities. Kds for metal cation solutions (20 μg/mg) on the amino sorbent were between 500-35000 mL/g. Both selectivity and sorption capabilities of the sorbents allow their use in environmental analysis and remediation.
中文翻译:

通过膦酸酯官能化的介孔二氧化硅的长颗粒清除污染金属
使用31 P-MAS-NMR,SEM-EDS,TEM,BET和分批吸附实验,在金属离子清除之前和/或之后(Bi +3,Bi ,3,3)进行了膦酸酯和/或双膦酸酯官能化的介孔二氧化硅(MS)吸附剂的开发和研究。 Ba +2,Cd +2,U +6,Ag +,Pd +2和Zn +2)。通过SEM-EDS确定吸附剂内部的磷含量,并且从NMR得出膦酸酯对双膦酸酯的含量。有机物负载量在15至38%w / w之间,与BET成反比。孔径(4.9-6.6 nm)和表面积(219-473 m 2(/ g)来自氮吸附等温线。膦酸酯的NMR信号被金属阳离子向下场偏移,而双膦酸酯的信号对于尿素衍生的吸附剂则向高场偏移,而对于氨基吸附剂的场频向低位偏移。吸附剂由微米尺寸的扁长颗粒组成。来自脲的吸附剂的扁长颗粒在吸附金属阳离子后是稳定的,而在氨基吸附剂的情况下,由于在碱性pH值下负电荷之间的排斥而被破坏。仅吸附具有较大离子直径的金属阳离子,这表明需要在吸附剂内部进行多点相互作用,这对于较小的阳离子是不可能的。对于所有吸附剂,铀酰的吸附数据符合Freundlich等温线模型,该模型预测了表面不均匀。从该模型得出的d值显示出良好的吸附能力。氨基吸附剂上金属阳离子溶液(20μg/ mg)的K d s在500-35000 mL / g之间。吸附剂的选择性和吸附能力均允许其在环境分析和修复中使用。



























 京公网安备 11010802027423号
京公网安备 11010802027423号