当前位置:
X-MOL 学术
›
J. Mol. Biol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Assembly of Tight Junction Strands: Claudin-10b and Claudin-3 Form Homo-Tetrameric Building Blocks that Polymerise in a Channel-Independent Manner.
Journal of Molecular Biology ( IF 5.6 ) Pub Date : 2020-03-04 , DOI: 10.1016/j.jmb.2020.02.034 C Hempel 1 , J Protze 2 , E Altun 1 , B Riebe 1 , A Piontek 2 , A Fromm 1 , I M Lee 1 , T Saleh 1 , D Günzel 1 , G Krause 2 , J Piontek 1
Journal of Molecular Biology ( IF 5.6 ) Pub Date : 2020-03-04 , DOI: 10.1016/j.jmb.2020.02.034 C Hempel 1 , J Protze 2 , E Altun 1 , B Riebe 1 , A Piontek 2 , A Fromm 1 , I M Lee 1 , T Saleh 1 , D Günzel 1 , G Krause 2 , J Piontek 1
Affiliation
|
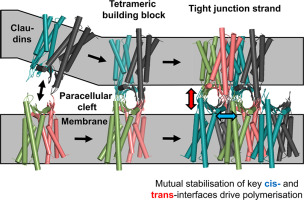
Tight junctions regulate paracellular permeability size and charge selectively. Models have been proposed for the molecular architecture of tight junction strands and paracellular channels. However, they are not fully consistent with experimental and structural data. Here, we analysed the architecture of claudin-based tight junction strands and channels by cellular reconstitution of strands, structure-guided mutagenesis, in silico protein docking and oligomer modelling. Prototypic channel- (Cldn10b) and barrier-forming (Cldn3) claudins were analysed. Förster resonance energy transfer (FRET) assays indicated multistep claudin polymerisation, starting with cis-oligomerization specific to the claudin subtype, followed by trans-interaction-triggered cis-polymerisation. Alternative protomer interfaces were modelled in silico and tested by cysteine-mediated crosslinking, confocal- and freeze fracture EM-based analysis of strand formation. The analysed claudin mutants included also mutations causing the HELIX syndrome. The results indicated that protomers in Cldn10b and Cldn3 strands form similar antiparallel double rows, as has been suggested for Cldn15. Mutually stabilising -hydrophilic and hydrophobic - cis- and trans-interfaces were identified that contained novel key residues of extracellular segments ECS1 and ECS2. Hydrophobic clustering of the flexible ECS1 β1β2 loops together with ECS2-ECS2 trans-interaction is suggested to be the driving force for conjunction of tetrameric building blocks into claudin polymers. Cldn10b and Cldn3 are indicated to share this polymerisation mechanism. However, in the paracellular centre of tetramers, electrostatic repulsion may lead to formation of pores (Cldn10b) and electrostatic attraction to barriers (Cldn3). Combining in vitro data and in silico modelling, this study improves mechanistic understanding of paracellular permeability regulation by elucidating claudin assembly and its pathologic alteration as in HELIX syndrome.
中文翻译:

紧密连接链的组装:Claudin-10b和Claudin-3形成均四聚体构建块,它们以独立于通道的方式聚合。
紧密连接调节细胞旁通透性大小并选择性充电。已经提出了用于紧密连接链和旁细胞通道的分子结构的模型。但是,它们与实验和结构数据并不完全一致。在这里,我们分析了基于claudin的紧密连接链和通道的结构,方法是通过链的细胞重构,结构引导的诱变,计算机蛋白对接和寡聚体建模。分析了原型通道-(Cldn10b)和屏障形成(Cldn3)的claudins。Förster共振能量转移(FRET)分析表明多步claudin聚合,从特定于claudin亚型的顺式低聚开始,然后是反式触发的顺式聚合。替代的protomer接口在计算机上建模,并通过半胱氨酸介导的交联,基于共聚焦和冷冻断裂的基于EM的链形成分析进行测试。分析的claudin突变体还包括引起HELIX综合征的突变。结果表明,Cldn10b和Cldn3链中的启动子形成相似的反平行双排,正如针对Cldn15所建议的那样。相互稳定的-亲水和疏水-顺式和反式接口被确定为包含细胞外片段ECS1和ECS2的新关键残基。弹性ECS1β1β2环的疏水性簇与ECS2-ECS2反式相互作用被认为是将四聚体结构单元结合成claudin聚合物的驱动力。表明Cldn10b和Cldn3具有这种聚合机理。然而,在四聚体的细胞旁中心,静电排斥作用可能导致孔(Cldn10b)的形成和对屏障(Cldn3)的静电吸引。结合体外数据和计算机模拟,该研究通过阐明claudin组装及其在HELIX综合征中的病理改变,提高了对细胞旁通透性调节的机制理解。
更新日期:2020-03-04
中文翻译:

紧密连接链的组装:Claudin-10b和Claudin-3形成均四聚体构建块,它们以独立于通道的方式聚合。
紧密连接调节细胞旁通透性大小并选择性充电。已经提出了用于紧密连接链和旁细胞通道的分子结构的模型。但是,它们与实验和结构数据并不完全一致。在这里,我们分析了基于claudin的紧密连接链和通道的结构,方法是通过链的细胞重构,结构引导的诱变,计算机蛋白对接和寡聚体建模。分析了原型通道-(Cldn10b)和屏障形成(Cldn3)的claudins。Förster共振能量转移(FRET)分析表明多步claudin聚合,从特定于claudin亚型的顺式低聚开始,然后是反式触发的顺式聚合。替代的protomer接口在计算机上建模,并通过半胱氨酸介导的交联,基于共聚焦和冷冻断裂的基于EM的链形成分析进行测试。分析的claudin突变体还包括引起HELIX综合征的突变。结果表明,Cldn10b和Cldn3链中的启动子形成相似的反平行双排,正如针对Cldn15所建议的那样。相互稳定的-亲水和疏水-顺式和反式接口被确定为包含细胞外片段ECS1和ECS2的新关键残基。弹性ECS1β1β2环的疏水性簇与ECS2-ECS2反式相互作用被认为是将四聚体结构单元结合成claudin聚合物的驱动力。表明Cldn10b和Cldn3具有这种聚合机理。然而,在四聚体的细胞旁中心,静电排斥作用可能导致孔(Cldn10b)的形成和对屏障(Cldn3)的静电吸引。结合体外数据和计算机模拟,该研究通过阐明claudin组装及其在HELIX综合征中的病理改变,提高了对细胞旁通透性调节的机制理解。


























 京公网安备 11010802027423号
京公网安备 11010802027423号