当前位置:
X-MOL 学术
›
J. Chem. Inf. Model.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
ON THE ASSESSMENT OF THE CRUZAIN CYSTEINE PROTEASE REVERSIBLE AND IRREVERSIBLE COVALENT INHIBITION MECHANISM.
Journal of Chemical Information and Modeling ( IF 5.6 ) Pub Date : 2020-03-03 , DOI: 10.1021/acs.jcim.9b01138 José Rogério A Silva 1 , Lorenzo Cianni 2 , Deborah Araujo 2 , Pedro Henrique Jatai Batista 2 , Daniela de Vita 2 , Fabiana Rosini 2 , Andrei Leitão 2 , Jerônimo Lameira 1 , Carlos A Montanari 2
Journal of Chemical Information and Modeling ( IF 5.6 ) Pub Date : 2020-03-03 , DOI: 10.1021/acs.jcim.9b01138 José Rogério A Silva 1 , Lorenzo Cianni 2 , Deborah Araujo 2 , Pedro Henrique Jatai Batista 2 , Daniela de Vita 2 , Fabiana Rosini 2 , Andrei Leitão 2 , Jerônimo Lameira 1 , Carlos A Montanari 2
Affiliation
|
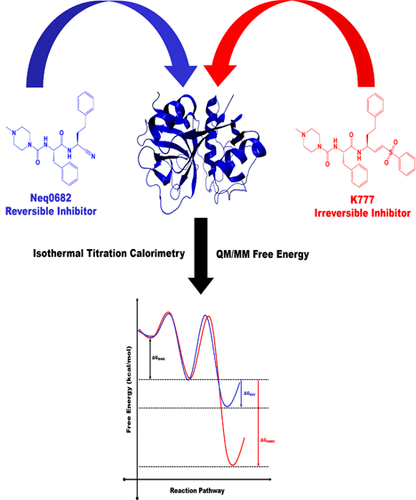
Reversible and irreversible covalent ligands are advanced cysteine protease inhibitors in the drug development pipeline. K777 is an irreversible inhibitor of cruzain, a necessary enzyme for the survival of the Trypanosoma cruzi (T. cruzi) parasite, the causative agent of Chagas disease. Despite their importance, irreversible covalent inhibitors are still often avoided due to the risk of adverse effects. Herein, we replaced the K777 vinyl sulfone group by nitrile moiety to obtain a reversible covalent inhibitor (Neq0682) of cysteine protease. Then, we used advanced experimental and computational techniques to explore details of the inhibition mechanism of cruzain by reversible and irreversible inhibitors. The isothermal titration calorimetry (ITC) analysis shows that inhibition of cruzain by irreversible inhibitor is thermodynamically more favorable than reversible one. The hybrid Quantum Mechanics/Molecular Mechanics (QM/MM) and Molecular Dynamics (MD) simulations were used to explore the mechanism of the reaction inhibition of cruzain by K777 and Neq0682. The calculated free energy profiles show that the Cys25 nucleophilic attack and His162 proton transfer occur in a single step for reversible inhibitor and two steps for irreversible covalent inhibitor. The hybrid QM/MM calculated free energies for the inhibition reaction correspond to -26.7 and -5.9 kcal mol-1 for K777 and Neq0682 at MP2/MM level, respectively. These results indicates the ΔG of reaction is very negative for the process involving K777, consequently, the covalent adduct cannot revert to noncovalent protein-ligand complex and its binding tends to be irreversible. Overall, the present study provides insights into covalent inhibition mechanism of cysteine proteases.
中文翻译:

克鲁斯半胱氨酸蛋白酶可逆和不可逆的价抑制机制的评估
可逆和不可逆共价配体是药物开发流程中先进的半胱氨酸蛋白酶抑制剂。K777是Cruzain的不可逆抑制剂,Cruzain是Chagas病的病原体Crupanosoma cruzi(T. cruzi)寄生虫生存的必需酶。尽管它们很重要,但由于存在不良反应的风险,仍然经常避免使用不可逆的共价抑制剂。在本文中,我们用腈基部分取代了K777乙烯基砜基团,以获得半胱氨酸蛋白酶的可逆共价抑制剂(Neq0682)。然后,我们使用了先进的实验和计算技术来探索可逆和不可逆抑制剂对Cruzain的抑制机理的细节。等温滴定热法(ITC)分析表明,不可逆抑制剂对克鲁萨因的抑制在热力学上比可逆抑制剂更有利。混合的量子力学/分子力学(QM / MM)和分子动力学(MD)模拟被用来探索K777和Neq0682抑制Cruzain的反应机理。计算出的自由能谱表明,对于可逆抑制剂,Cys25亲核攻击和His162质子转移一步发生,对于不可逆共价抑制剂,两步发生。杂化QM / MM计算的抑制反应自由能分别对应于MP2 / MM水平的K777和Neq0682的-26.7和-5.9 kcal mol-1。这些结果表明,对于涉及K777的过程,反应的ΔG非常负,因此,共价加合物不能还原为非共价蛋白-配体复合物,其结合趋于不可逆。总体而言,本研究提供了对半胱氨酸蛋白酶的共价抑制机制的见解。
更新日期:2020-04-24
中文翻译:

克鲁斯半胱氨酸蛋白酶可逆和不可逆的价抑制机制的评估
可逆和不可逆共价配体是药物开发流程中先进的半胱氨酸蛋白酶抑制剂。K777是Cruzain的不可逆抑制剂,Cruzain是Chagas病的病原体Crupanosoma cruzi(T. cruzi)寄生虫生存的必需酶。尽管它们很重要,但由于存在不良反应的风险,仍然经常避免使用不可逆的共价抑制剂。在本文中,我们用腈基部分取代了K777乙烯基砜基团,以获得半胱氨酸蛋白酶的可逆共价抑制剂(Neq0682)。然后,我们使用了先进的实验和计算技术来探索可逆和不可逆抑制剂对Cruzain的抑制机理的细节。等温滴定热法(ITC)分析表明,不可逆抑制剂对克鲁萨因的抑制在热力学上比可逆抑制剂更有利。混合的量子力学/分子力学(QM / MM)和分子动力学(MD)模拟被用来探索K777和Neq0682抑制Cruzain的反应机理。计算出的自由能谱表明,对于可逆抑制剂,Cys25亲核攻击和His162质子转移一步发生,对于不可逆共价抑制剂,两步发生。杂化QM / MM计算的抑制反应自由能分别对应于MP2 / MM水平的K777和Neq0682的-26.7和-5.9 kcal mol-1。这些结果表明,对于涉及K777的过程,反应的ΔG非常负,因此,共价加合物不能还原为非共价蛋白-配体复合物,其结合趋于不可逆。总体而言,本研究提供了对半胱氨酸蛋白酶的共价抑制机制的见解。


























 京公网安备 11010802027423号
京公网安备 11010802027423号