当前位置:
X-MOL 学术
›
J. Nat. Prod.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Polyacetylenes from Oplopanax horridus and Panax ginseng: Relationship between Structure and PPARγ Activation.
Journal of Natural Products ( IF 5.1 ) Pub Date : 2020-03-04 , DOI: 10.1021/acs.jnatprod.9b00691 Mirta Resetar 1 , Xin Liu 2 , Sonja Herdlinger 3 , Olaf Kunert 4 , Eva-Maria Pferschy-Wenzig 2 , Simone Latkolik 1 , Theresa Steinacher 3 , Daniela Schuster 5 , Rudolf Bauer 2 , Verena M Dirsch 1
Journal of Natural Products ( IF 5.1 ) Pub Date : 2020-03-04 , DOI: 10.1021/acs.jnatprod.9b00691 Mirta Resetar 1 , Xin Liu 2 , Sonja Herdlinger 3 , Olaf Kunert 4 , Eva-Maria Pferschy-Wenzig 2 , Simone Latkolik 1 , Theresa Steinacher 3 , Daniela Schuster 5 , Rudolf Bauer 2 , Verena M Dirsch 1
Affiliation
|
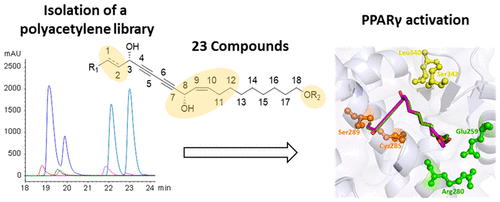
Oplopanax horridus and Panax ginseng are members of the plant family Araliaceae, which is rich in structurally diverse polyacetylenes. In this work, we isolated and determined structures of 23 aliphatic C17 and C18 polyacetylenes, of which five are new compounds. Polyacetylenes have a suitable scaffold for binding to PPARγ, a ligand-activated transcription factor involved in metabolic regulation. Using a reporter gene assay, their potential was investigated to activate PPARγ. The majority of the polyacetylenes showed at least some PPARγ activity, among which oplopantriol B 18-acetate (1) and oplopantriol B (2) were the most potent partial PPARγ activators. By employing in silico molecular docking and comparing the activities of structural analogues, features are described that are involved in PPARγ activation, as well as in cytotoxicity. It was found that the type of C-1 to C-2 bond, the polarity of the terminal alkyl chain, and the backbone flexibility can impact bioactivity of polyacetylenes, while diol structures with a C-1 to C-2 double bond showed enhanced cytotoxicity. Since PPARγ activators have antidiabetic and anti-inflammatory properties, the present results may help explain some of the beneficial effects observed in the traditional use of O. horridus extracts. Additionally, they might guide the polyacetylene-based design of future PPARγ partial agonists.
中文翻译:

lop和人参中的聚乙炔:结构与PPARγ活化之间的关系。
lop(Olopopanax horridus)和人参(Panax ginseng)是植物科(Araliaceae)的成员,该科富含结构多样的聚乙炔。在这项工作中,我们分离并确定了23种脂肪族C17和C18聚乙炔的结构,其中五个是新化合物。聚乙炔具有适合与PPARγ结合的支架,PPARγ是参与代谢调节的配体激活的转录因子。使用报告基因测定法,研究了它们激活PPARγ的潜力。大多数聚乙炔具有至少一些PPARγ活性,其中oplopantriol B 18-乙酸盐(1)和oplopantriol B(2)是最有效的部分PPARγ活化剂。通过计算机内分子对接并比较结构类似物的活性,描述了参与PPARγ活化以及细胞毒性的特征。发现C-1至C-2键的类型,末端烷基链的极性和主链柔性会影响聚乙炔的生物活性,而具有C-1至C-2双键的二醇结构显示出增强的结构。细胞毒性。由于PPARγ激活剂具有抗糖尿病和抗发炎的特性,因此本发明的结果可能有助于解释在传统使用O. horridus提取物中观察到的一些有益作用。此外,它们可能指导未来PPARγ部分激动剂的基于聚乙炔的设计。目前的结果可能有助于解释在传统使用O. horridus提取物中观察到的一些有益效果。此外,它们可能指导未来PPARγ部分激动剂的基于聚乙炔的设计。目前的结果可能有助于解释在传统使用O. horridus提取物中观察到的一些有益效果。另外,它们可能指导未来PPARγ部分激动剂的基于聚乙炔的设计。
更新日期:2020-03-04
中文翻译:

lop和人参中的聚乙炔:结构与PPARγ活化之间的关系。
lop(Olopopanax horridus)和人参(Panax ginseng)是植物科(Araliaceae)的成员,该科富含结构多样的聚乙炔。在这项工作中,我们分离并确定了23种脂肪族C17和C18聚乙炔的结构,其中五个是新化合物。聚乙炔具有适合与PPARγ结合的支架,PPARγ是参与代谢调节的配体激活的转录因子。使用报告基因测定法,研究了它们激活PPARγ的潜力。大多数聚乙炔具有至少一些PPARγ活性,其中oplopantriol B 18-乙酸盐(1)和oplopantriol B(2)是最有效的部分PPARγ活化剂。通过计算机内分子对接并比较结构类似物的活性,描述了参与PPARγ活化以及细胞毒性的特征。发现C-1至C-2键的类型,末端烷基链的极性和主链柔性会影响聚乙炔的生物活性,而具有C-1至C-2双键的二醇结构显示出增强的结构。细胞毒性。由于PPARγ激活剂具有抗糖尿病和抗发炎的特性,因此本发明的结果可能有助于解释在传统使用O. horridus提取物中观察到的一些有益作用。此外,它们可能指导未来PPARγ部分激动剂的基于聚乙炔的设计。目前的结果可能有助于解释在传统使用O. horridus提取物中观察到的一些有益效果。此外,它们可能指导未来PPARγ部分激动剂的基于聚乙炔的设计。目前的结果可能有助于解释在传统使用O. horridus提取物中观察到的一些有益效果。另外,它们可能指导未来PPARγ部分激动剂的基于聚乙炔的设计。



























 京公网安备 11010802027423号
京公网安备 11010802027423号