当前位置:
X-MOL 学术
›
CrystEngComm
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
New isomeric Ni(NCS)2 coordination compounds: crystal structures, magnetic properties as well as ex situ and in situ investigations on their synthesis and transition behaviour
CrystEngComm ( IF 3.1 ) Pub Date : 2020/03/03 , DOI: 10.1039/d0ce00181c Carsten Wellm 1, 2, 3, 4 , Tristan Neumann 1, 2, 3, 4 , Magdalena Ceglarska 5, 6, 7, 8 , Gianpiero Gallo 4, 9, 10, 11, 12 , Michał Rams 5, 6, 7, 8 , Robert E. Dinnebier 4, 9, 10 , Christian Näther 1, 2, 3, 4
CrystEngComm ( IF 3.1 ) Pub Date : 2020/03/03 , DOI: 10.1039/d0ce00181c Carsten Wellm 1, 2, 3, 4 , Tristan Neumann 1, 2, 3, 4 , Magdalena Ceglarska 5, 6, 7, 8 , Gianpiero Gallo 4, 9, 10, 11, 12 , Michał Rams 5, 6, 7, 8 , Robert E. Dinnebier 4, 9, 10 , Christian Näther 1, 2, 3, 4
Affiliation
|
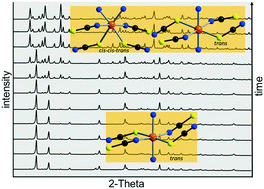
The reaction of Ni(NCS)2 with 3-ethylpyridine in water leads to the formation of two known modifications of Ni(NCS)2(3-ethylpyridine)4 (1-I and 1-II) as well as of Ni(NCS)2(3-ethylpyridine)2(H2O)2 (2) that consist of discrete complexes. If the synthesis is performed in ethyl acetate, the new compound [Ni(NCS)2(3-ethylpyridine)2]n (3-lc) formed that comprises linear and corrugated Ni(NCS)2 chains in one crystal. By changing the solvent to dichloromethane, a solvate of compound 3 is obtained that consists exclusively of corrugated chains (3c-CH2Cl2). 3c-CH2Cl2 is unstable and transforms immediately into 3-lc. Upon heating the precursor complexes 1-I, 1-II and 2, the 3-ethylpyridine ligands are removed in two steps leading to a mixture of 3-lc and an additional isomer of 3 (3-l) in the first step that decomposes upon further heating into a compound with the composition [Ni(NCS)2(3-ethylpyridine)]n (4). Ex and in situ XRPD measurements reveal that a mixture of two modifications (4-I and 4-II) is always formed, preventing their structure determination by XRPD. Time dependent investigations in solution prove that 3-l is formed under kinetic control and transforms into 3-lc within several hours that is thermodynamically stable at room-temperature. Structure analysis of 3-l shows that in contrast to 3-lc and 3c-CH2Cl2 this isomer consists exclusively of linear Ni(NCS)2 chains. Magnetic measurements of the new chain compounds 3-l and 3-lc shows χT values that are typical for Ni(II) cations with S = 1. For 3-l the exchange constant was determined by fitting of the magnetic data leading to a value that is similar to that of [Ni(NSC)2(2,2′-bipyridine)2]n.
中文翻译:

新的异构Ni(NCS)2配位化合物:晶体结构,磁性以及合成和过渡行为的原位和原位研究
的Ni(NCS)的反应2与3-乙基吡啶在水导致的(NCS)的Ni的两种已知的修改的形成2(3-乙基吡啶)4(1 -I和1 -II NCS)以及镍( )2(3-乙基吡啶)2(H 2 O)2(2),由不连续的络合物组成。如果在乙酸乙酯中进行合成,则形成的新化合物[Ni(NCS)2(3-乙基吡啶)2 ] n(3 -lc)包含线性和波纹状Ni(NCS)2一条水晶链。通过将溶剂改变为二氯甲烷,获得化合物3的溶剂化物,其仅由波纹链(3c- CH 2 Cl 2)组成。3c -CH 2 Cl 2不稳定,立即变为3 -lc。当加热前体复合物1 -I,1 -II和2,3-乙基吡啶配体在两个步骤中导致的混合物中除去3 -lc和额外的异构体3(3 -l)在第一步中分解,然后进一步加热分解为组成为[Ni(NCS)2(3-乙基吡啶)] n(4)的化合物。离和原位XRPD测量表明,两种修饰(混合物4 -I和4 -II)总是形成,防止其结构测定通过XRPD。在溶液中随时间变化的调查证明3 -l被动力学控制和变换下形成为3 -lc数小时即热力学在室温下稳定的范围内。3- l的结构分析与3 -lc和3c -CH 2 Cl 2相比,该异构体仅由线性Ni(NCS)2链组成。新链化合物3 -l和3 -lc的磁性测量显示χT值,典型值为S = 1的Ni(II)阳离子。对于3 -1,交换常数是通过拟合磁性数据得出的,从而得出一个值。与[Ni(NSC)2(2,2'-联吡啶)2 ] n相似。
更新日期:2020-03-30
中文翻译:

新的异构Ni(NCS)2配位化合物:晶体结构,磁性以及合成和过渡行为的原位和原位研究
的Ni(NCS)的反应2与3-乙基吡啶在水导致的(NCS)的Ni的两种已知的修改的形成2(3-乙基吡啶)4(1 -I和1 -II NCS)以及镍( )2(3-乙基吡啶)2(H 2 O)2(2),由不连续的络合物组成。如果在乙酸乙酯中进行合成,则形成的新化合物[Ni(NCS)2(3-乙基吡啶)2 ] n(3 -lc)包含线性和波纹状Ni(NCS)2一条水晶链。通过将溶剂改变为二氯甲烷,获得化合物3的溶剂化物,其仅由波纹链(3c- CH 2 Cl 2)组成。3c -CH 2 Cl 2不稳定,立即变为3 -lc。当加热前体复合物1 -I,1 -II和2,3-乙基吡啶配体在两个步骤中导致的混合物中除去3 -lc和额外的异构体3(3 -l)在第一步中分解,然后进一步加热分解为组成为[Ni(NCS)2(3-乙基吡啶)] n(4)的化合物。离和原位XRPD测量表明,两种修饰(混合物4 -I和4 -II)总是形成,防止其结构测定通过XRPD。在溶液中随时间变化的调查证明3 -l被动力学控制和变换下形成为3 -lc数小时即热力学在室温下稳定的范围内。3- l的结构分析与3 -lc和3c -CH 2 Cl 2相比,该异构体仅由线性Ni(NCS)2链组成。新链化合物3 -l和3 -lc的磁性测量显示χT值,典型值为S = 1的Ni(II)阳离子。对于3 -1,交换常数是通过拟合磁性数据得出的,从而得出一个值。与[Ni(NSC)2(2,2'-联吡啶)2 ] n相似。



























 京公网安备 11010802027423号
京公网安备 11010802027423号