当前位置:
X-MOL 学术
›
Org. Chem. Front.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Palladium-catalyzed cross-coupling of (hetero)aryl or alkenyl sulfonates with aryl titanium as the multi-functional reagent
Organic Chemistry Frontiers ( IF 5.4 ) Pub Date : 2020-03-03 , DOI: 10.1039/c9qo01537j Hang Wai Lee 1, 2, 3, 4, 5 , Chau Ming So 1, 2, 3, 4, 5 , On Ying Yuen 1, 2, 3, 4, 6 , Wing Tak Wong 1, 2, 3, 4, 5 , Fuk Yee Kwong 1, 2, 3, 4, 6
Organic Chemistry Frontiers ( IF 5.4 ) Pub Date : 2020-03-03 , DOI: 10.1039/c9qo01537j Hang Wai Lee 1, 2, 3, 4, 5 , Chau Ming So 1, 2, 3, 4, 5 , On Ying Yuen 1, 2, 3, 4, 6 , Wing Tak Wong 1, 2, 3, 4, 5 , Fuk Yee Kwong 1, 2, 3, 4, 6
Affiliation
|
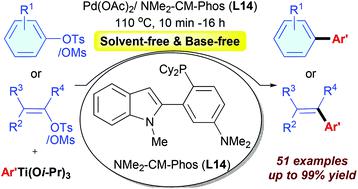
The first palladium-catalyzed cross-coupling reaction of aryl/heteroaryl and alkenyl mesylates and tosylates with aryl titanium as the multi-functional reagent is reported. Using the catalyst system of Pd(OAc)2 associated with the new NMe2-CM-Phos (L14), a broad range of electron-rich, electron-neutral, electron-deficient, and sterically hindered aryl/heteroaryl and alkenyl mesylates and tosylates are well coupled with aryl titanium reagents to give the corresponding products in good to excellent yields. The catalyst loading down to 0.2 mol% Pd and the reaction time shortening to 10 min can be achieved. The reaction can be easily scaled up to the gram scale without diminishing the product yield.
中文翻译:

钯催化的(杂)芳基或烯基磺酸盐与芳基钛作为多功能试剂的交叉偶联
报道了芳基/杂芳基与烯基甲磺酸酯和甲苯磺酸酯与芳族钛作为多功能试剂的第一个钯催化的交叉偶联反应。使用与新型NMe 2 -CM-Phos(L14)缔合的Pd(OAc)2催化剂体系,可以得到多种富电子,电子中性,电子不足和空间受阻的芳基/杂芳基和烯基甲磺酸酯,甲苯磺酸酯与芳基钛试剂良好偶联,以良好至优异的产率得到相应的产物。可以实现低至0.2 mol%Pd的催化剂负载量和缩短至10分钟的反应时间。该反应可以容易地放大至克级,而不会降低产物的产率。
更新日期:2020-04-24
中文翻译:

钯催化的(杂)芳基或烯基磺酸盐与芳基钛作为多功能试剂的交叉偶联
报道了芳基/杂芳基与烯基甲磺酸酯和甲苯磺酸酯与芳族钛作为多功能试剂的第一个钯催化的交叉偶联反应。使用与新型NMe 2 -CM-Phos(L14)缔合的Pd(OAc)2催化剂体系,可以得到多种富电子,电子中性,电子不足和空间受阻的芳基/杂芳基和烯基甲磺酸酯,甲苯磺酸酯与芳基钛试剂良好偶联,以良好至优异的产率得到相应的产物。可以实现低至0.2 mol%Pd的催化剂负载量和缩短至10分钟的反应时间。该反应可以容易地放大至克级,而不会降低产物的产率。


























 京公网安备 11010802027423号
京公网安备 11010802027423号