当前位置:
X-MOL 学术
›
Chem. Sci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Excited-state proton-coupled electron transfer within ion pairs
Chemical Science ( IF 8.4 ) Pub Date : 2020/03/03 , DOI: 10.1039/c9sc04941j Wesley B. Swords 1, 2, 3, 4, 5 , Gerald J. Meyer 1, 6, 7, 8 , Leif Hammarström 1, 2, 3, 4, 5
Chemical Science ( IF 8.4 ) Pub Date : 2020/03/03 , DOI: 10.1039/c9sc04941j Wesley B. Swords 1, 2, 3, 4, 5 , Gerald J. Meyer 1, 6, 7, 8 , Leif Hammarström 1, 2, 3, 4, 5
Affiliation
|
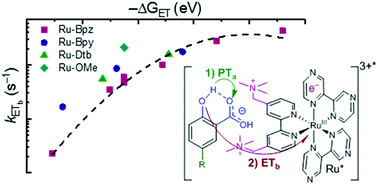
The use of light to drive proton-coupled electron transfer (PCET) reactions has received growing interest, with recent focus on the direct use of excited states in PCET reactions (ES-PCET). Electrostatic ion pairs provide a scaffold to reduce reaction orders and have facilitated many discoveries in electron-transfer chemistry. Their use, however, has not translated to PCET. Herein, we show that ion pairs, formed solely through electrostatic interactions, provide a general, facile means to study an ES-PCET mechanism. These ion pairs formed readily between salicylate anions and tetracationic ruthenium complexes in acetonitrile solution. Upon light excitation, quenching of the ruthenium excited state occurred through ES-PCET oxidation of salicylate within the ion pair. Transient absorption spectroscopy identified the reduced ruthenium complex and oxidized salicylate radical as the primary photoproducts of this reaction. The reduced reaction order due to ion pairing allowed the first-order PCET rate constants to be directly measured through nanosecond photoluminescence spectroscopy. These PCET rate constants saturated at larger driving forces consistent with approaching the Marcus barrierless region. Surprisingly, a proton-transfer tautomer of salicylate, with the proton localized on the carboxylate functional group, was present in acetonitrile. A pre-equilibrium model based on this tautomerization provided non-adiabatic electron-transfer rate constants that were well described by Marcus theory. Electrostatic ion pairs were critical to our ability to investigate this PCET mechanism without the need to covalently link the donor and acceptor or introduce specific hydrogen bonding sites that could compete in alternate PCET pathways.
中文翻译:

离子对内激发态质子耦合电子的转移
使用光来驱动质子偶联电子转移(PCET)反应已引起越来越多的兴趣,最近集中在PCET反应(ES-PCET)中直接使用激发态。静电离子对提供了一种降低反应顺序的支架,并促进了电子转移化学中的许多发现。但是,它们的使用尚未转换为PCET。在本文中,我们表明,仅通过静电相互作用形成的离子对提供了研究ES-PCET机制的通用且简便的方法。这些离子对很容易在乙腈溶液中的水杨酸根阴离子与四阳离子钌络合物之间形成。在光激发后,通过离子对内水杨酸酯的ES-PCET氧化,发生了钌激发态的猝灭。瞬态吸收光谱法鉴定出还原的钌络合物和氧化的水杨酸盐自由基是该反应的主要光产物。由于离子配对而降低的反应阶数使得可以通过纳秒光致发光光谱法直接测量一阶PCET速率常数。这些PCET速率常数在较大的驱动力下达到饱和,这与接近Marcus无障碍区域一致。令人惊讶地,乙腈中存在水杨酸酯的质子转移互变异构体,质子位于羧酸酯官能团上。基于这种互变异构的预平衡模型提供了非绝热电子传输速率常数,Marcus理论很好地描述了该常数。
更新日期:2020-04-01
中文翻译:

离子对内激发态质子耦合电子的转移
使用光来驱动质子偶联电子转移(PCET)反应已引起越来越多的兴趣,最近集中在PCET反应(ES-PCET)中直接使用激发态。静电离子对提供了一种降低反应顺序的支架,并促进了电子转移化学中的许多发现。但是,它们的使用尚未转换为PCET。在本文中,我们表明,仅通过静电相互作用形成的离子对提供了研究ES-PCET机制的通用且简便的方法。这些离子对很容易在乙腈溶液中的水杨酸根阴离子与四阳离子钌络合物之间形成。在光激发后,通过离子对内水杨酸酯的ES-PCET氧化,发生了钌激发态的猝灭。瞬态吸收光谱法鉴定出还原的钌络合物和氧化的水杨酸盐自由基是该反应的主要光产物。由于离子配对而降低的反应阶数使得可以通过纳秒光致发光光谱法直接测量一阶PCET速率常数。这些PCET速率常数在较大的驱动力下达到饱和,这与接近Marcus无障碍区域一致。令人惊讶地,乙腈中存在水杨酸酯的质子转移互变异构体,质子位于羧酸酯官能团上。基于这种互变异构的预平衡模型提供了非绝热电子传输速率常数,Marcus理论很好地描述了该常数。



























 京公网安备 11010802027423号
京公网安备 11010802027423号