当前位置:
X-MOL 学术
›
Cell Death Dis.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Retinal ganglion cell loss in kinesin-1 cargo Alcadein α deficient mice.
Cell Death & Disease ( IF 9 ) Pub Date : 2020-03-03 , DOI: 10.1038/s41419-020-2363-x Yuki Nakano 1 , Kazuyuki Hirooka 2 , Yoichi Chiba 3 , Masaki Ueno 3 , Daiki Ojima 4 , Md Razib Hossain 4 , Hiroo Takahashi 4 , Tohru Yamamoto 4 , Yoshiaki Kiuchi 2
Cell Death & Disease ( IF 9 ) Pub Date : 2020-03-03 , DOI: 10.1038/s41419-020-2363-x Yuki Nakano 1 , Kazuyuki Hirooka 2 , Yoichi Chiba 3 , Masaki Ueno 3 , Daiki Ojima 4 , Md Razib Hossain 4 , Hiroo Takahashi 4 , Tohru Yamamoto 4 , Yoshiaki Kiuchi 2
Affiliation
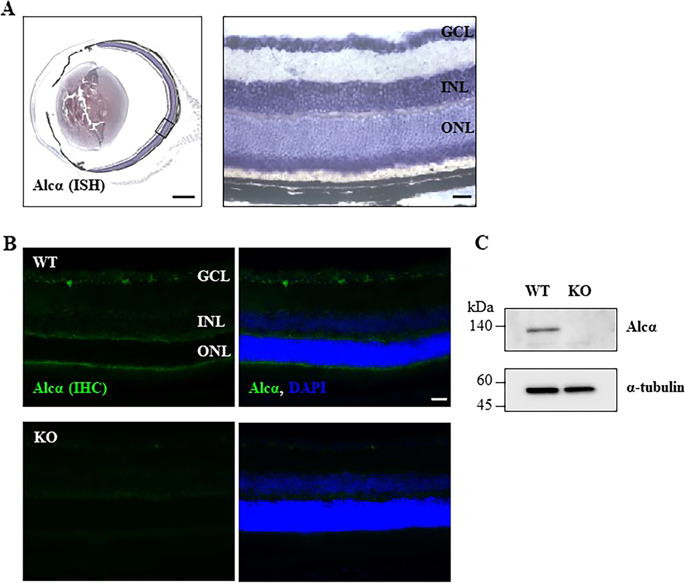
|
Maintenance of retinal ganglion cells (RGCs) activity is relied on axonal transport conveying materials required for their survival such as neurotrophic factors. Kinesin-1 undergoes anterograde transport in axons, and Alcadein α (Alcα; also called calsyntenin-1) is a major cargo adaptor protein that can drive kinesin-1 to transport vesicles containing Alcα. The long-term effects of Alcα-deficiency on retinal morphology and survival of RGCs during postnatal development were examined in Alcα knockout mice. At 1.5, 3, 6, and 15 months postnatal, the number of retrogradely labeled RGCs was determined in flat-mounted retinas of Alcα-deficient and wild-type mice. Retinal damage was assessed histologically by determining the retinal thickness. Intraocular pressure (IOP) was measured with a Tonolab tonometer. At 1.5 months postnatal, the number of retrogradely labeled RGCs was not different between wild-type and Alcα-deficient mice. However, at 3, 6, and 15 months postnatal, the number of RGCs was significantly lower in Alcα deficient mice than those of wild-type mice (143 ± 41.1 cells/mm2 vs. 208 ± 28.4 cells/mm2, respectively, at 3 months; P < 0.01). No differences were seen in retinal thickness or IOP between the two types of mice at any postnatal age. Alcα-deficient mice showed spontaneous loss of RGCs but no elevation in IOP. These mice mimic normal-tension glaucoma and will be useful for investigating the mechanism of neurodegeneration in this disorder and for developing treatments for RGC loss that does not involve changes in IOP.
中文翻译:

驱动蛋白1货Alcadeinα缺陷小鼠的视网膜神经节细胞损失。
视网膜神经节细胞(RGCs)活性的维持依赖于其生存所需的轴突运输材料,例如神经营养因子。Kinesin-1在轴突中经历顺行转运,而Alcadeinα(Alcα;也称为Calsyntenin-1)是一种主要的货运衔接蛋白,可以驱动kinesin-1转运含有Alcα的囊泡。在Alcα基因敲除小鼠中检查了Alcα缺乏对视网膜形态和产后发育期间RGC存活的长期影响。在出生后1.5、3、6和15个月,在Alcα缺陷型和野生型小鼠的平置视网膜中确定逆行标记的RGC的数量。通过确定视网膜厚度在组织学上评估视网膜损伤。用Tonolab眼压计测量眼内压(IOP)。产后1.5个月,逆行标记的RGC的数量在野生型和缺乏Alcα的小鼠之间没有差异。然而,在出生后3、6和15个月,缺乏Alcα的小鼠的RGC数量显着低于野生型小鼠(分别为143±41.1细胞/ mm2与208±28.4细胞/ mm2,在3月; P <0.01)。在任何出生年龄后,两种类型的小鼠的视网膜厚度或眼压均无差异。缺乏Alcα的小鼠表现出自发性RGC丢失,但IOP没有升高。这些小鼠模仿正常血压的青光眼,将可用于研究这种疾病的神经退行性机制,以及开发不涉及IOP改变的RGC丧失的治疗方法。Alcα缺陷型小鼠的RGC数量显着低于野生型小鼠(3个月时分别为143±41.1细胞/ mm2与208±28.4细胞/ mm2; P <0.01)。在任何出生年龄后,两种类型的小鼠的视网膜厚度或眼压均无差异。缺乏Alcα的小鼠表现出自发性RGC丢失,但IOP没有升高。这些小鼠模仿正常血压的青光眼,将可用于研究这种疾病的神经退行性机制,以及开发不涉及IOP改变的RGC丧失的治疗方法。Alcα缺陷型小鼠的RGC数量显着低于野生型小鼠(3个月时分别为143±41.1细胞/ mm2与208±28.4细胞/ mm2; P <0.01)。在任何出生年龄后,两种类型的小鼠的视网膜厚度或眼压均无差异。缺乏Alcα的小鼠表现出自发性RGC丢失,但IOP没有升高。这些小鼠模仿正常血压的青光眼,将有助于研究这种疾病的神经退行性机制,以及开发不涉及IOP改变的RGC丧失的治疗方法。缺乏Alcα的小鼠表现出自发性RGC丢失,但IOP没有升高。这些小鼠模仿正常血压的青光眼,将可用于研究这种疾病的神经退行性机制,以及开发不涉及IOP改变的RGC丧失的治疗方法。缺乏Alcα的小鼠表现出自发性RGC丢失,但IOP没有升高。这些小鼠模仿正常血压的青光眼,将可用于研究这种疾病的神经退行性机制,以及开发不涉及IOP改变的RGC丧失的治疗方法。
更新日期:2020-03-03
中文翻译:

驱动蛋白1货Alcadeinα缺陷小鼠的视网膜神经节细胞损失。
视网膜神经节细胞(RGCs)活性的维持依赖于其生存所需的轴突运输材料,例如神经营养因子。Kinesin-1在轴突中经历顺行转运,而Alcadeinα(Alcα;也称为Calsyntenin-1)是一种主要的货运衔接蛋白,可以驱动kinesin-1转运含有Alcα的囊泡。在Alcα基因敲除小鼠中检查了Alcα缺乏对视网膜形态和产后发育期间RGC存活的长期影响。在出生后1.5、3、6和15个月,在Alcα缺陷型和野生型小鼠的平置视网膜中确定逆行标记的RGC的数量。通过确定视网膜厚度在组织学上评估视网膜损伤。用Tonolab眼压计测量眼内压(IOP)。产后1.5个月,逆行标记的RGC的数量在野生型和缺乏Alcα的小鼠之间没有差异。然而,在出生后3、6和15个月,缺乏Alcα的小鼠的RGC数量显着低于野生型小鼠(分别为143±41.1细胞/ mm2与208±28.4细胞/ mm2,在3月; P <0.01)。在任何出生年龄后,两种类型的小鼠的视网膜厚度或眼压均无差异。缺乏Alcα的小鼠表现出自发性RGC丢失,但IOP没有升高。这些小鼠模仿正常血压的青光眼,将可用于研究这种疾病的神经退行性机制,以及开发不涉及IOP改变的RGC丧失的治疗方法。Alcα缺陷型小鼠的RGC数量显着低于野生型小鼠(3个月时分别为143±41.1细胞/ mm2与208±28.4细胞/ mm2; P <0.01)。在任何出生年龄后,两种类型的小鼠的视网膜厚度或眼压均无差异。缺乏Alcα的小鼠表现出自发性RGC丢失,但IOP没有升高。这些小鼠模仿正常血压的青光眼,将可用于研究这种疾病的神经退行性机制,以及开发不涉及IOP改变的RGC丧失的治疗方法。Alcα缺陷型小鼠的RGC数量显着低于野生型小鼠(3个月时分别为143±41.1细胞/ mm2与208±28.4细胞/ mm2; P <0.01)。在任何出生年龄后,两种类型的小鼠的视网膜厚度或眼压均无差异。缺乏Alcα的小鼠表现出自发性RGC丢失,但IOP没有升高。这些小鼠模仿正常血压的青光眼,将有助于研究这种疾病的神经退行性机制,以及开发不涉及IOP改变的RGC丧失的治疗方法。缺乏Alcα的小鼠表现出自发性RGC丢失,但IOP没有升高。这些小鼠模仿正常血压的青光眼,将可用于研究这种疾病的神经退行性机制,以及开发不涉及IOP改变的RGC丧失的治疗方法。缺乏Alcα的小鼠表现出自发性RGC丢失,但IOP没有升高。这些小鼠模仿正常血压的青光眼,将可用于研究这种疾病的神经退行性机制,以及开发不涉及IOP改变的RGC丧失的治疗方法。



























 京公网安备 11010802027423号
京公网安备 11010802027423号