当前位置:
X-MOL 学术
›
Cell Death Dis.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Hypoxia-induced lncRNA PDIA3P1 promotes mesenchymal transition via sponging of miR-124-3p in glioma.
Cell Death & Disease ( IF 9 ) Pub Date : 2020-03-03 , DOI: 10.1038/s41419-020-2345-z Shaobo Wang 1, 2, 3 , Yanhua Qi 1, 2, 3 , Xiao Gao 1, 2, 3 , Wei Qiu 1, 2, 3 , Qinglin Liu 1, 4 , Xiaofan Guo 1, 2, 3 , Mingyu Qian 1, 2, 3 , Zihang Chen 1, 2, 3 , Zongpu Zhang 1, 2, 3 , Huizhi Wang 1, 2, 3 , Jianye Xu 1, 2, 3 , Hao Xue 1, 2, 3 , Xing Guo 1, 2, 3 , Ping Zhang 1, 2, 3 , Rongrong Zhao 1, 2, 3 , Gang Li 1, 2, 3
Cell Death & Disease ( IF 9 ) Pub Date : 2020-03-03 , DOI: 10.1038/s41419-020-2345-z Shaobo Wang 1, 2, 3 , Yanhua Qi 1, 2, 3 , Xiao Gao 1, 2, 3 , Wei Qiu 1, 2, 3 , Qinglin Liu 1, 4 , Xiaofan Guo 1, 2, 3 , Mingyu Qian 1, 2, 3 , Zihang Chen 1, 2, 3 , Zongpu Zhang 1, 2, 3 , Huizhi Wang 1, 2, 3 , Jianye Xu 1, 2, 3 , Hao Xue 1, 2, 3 , Xing Guo 1, 2, 3 , Ping Zhang 1, 2, 3 , Rongrong Zhao 1, 2, 3 , Gang Li 1, 2, 3
Affiliation
|
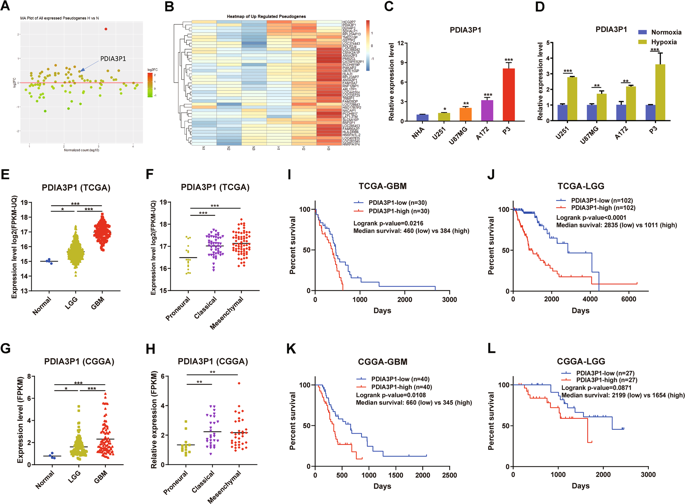
Hypoxia is a critical factor in the malignant progression of glioma, especially for the highly-invasive mesenchymal (MES) subtype. But the detailed mechanisms in hypoxia-induced glioma MES transition remain elusive. Pseudogenes, once considered to be non-functional relics of evolution, are emerging as a critical factor in human tumorigenesis and progression. Here, we investigated the clinical significance, biological function, and mechanisms of protein disulfide isomerase family A member 3 pseudogene 1 (PDIA3P1) in hypoxia-induced glioma MES transition. In this study, we found that PDIA3P1 expression was closely related to tumor degree, transcriptome subtype, and prognosis in glioma patients. Enrichment analysis found that high PDIA3P1 expression was associated with epithelial-mesenchymal transition, extracellular matrix (ECM) disassembly, and angiogenesis. In vitro study revealed that overexpression of PDIA3P1 enhanced the migration and invasion capacity of glioma cells, while knockdown of PDIA3P1 induced the opposite effect. Further studies revealed that PDIA3P1 functions as a ceRNA, sponging miR-124-3p to modulate RELA expression and activate the downstream NF-κB pathway, thus promoting the MES transition of glioma cells. In addition, Hypoxia Inducible Factor 1 was confirmed to directly bind to the PDIA3P1 promotor region and activate its transcription. In conclusion, PDIA3P1 is a crucial link between hypoxia and glioma MES transition through the PDIA3P1-miR-124-3p-RELA axis, which may serve as a prognostic indicator and potential therapeutic target for glioma treatment.
中文翻译:

缺氧诱导的lncRNA PDIA3P1通过胶质瘤中miR-124-3p的海绵化促进间质转化。
缺氧是神经胶质瘤恶性进展的关键因素,尤其是对于高度侵入性的间充质(MES)亚型。但是在缺氧诱导的神经胶质瘤MES转移的详细机制仍不清楚。伪基因曾经被认为是进化的非功能性遗迹,正在成为人类肿瘤发生和发展的关键因素。在这里,我们调查了缺氧诱导的神经胶质瘤MES转换中蛋白质二硫键异构酶家族A成员3假基因1(PDIA3P1)的临床意义,生物学功能和机制。在这项研究中,我们发现PDIA3P1的表达与神经胶质瘤患者的肿瘤程度,转录组亚型和预后密切相关。富集分析发现PDIA3P1高表达与上皮-间质转化,细胞外基质(ECM)拆卸,和血管生成。体外研究表明,PDIA3P1的过表达增强了胶质瘤细胞的迁移和侵袭能力,而敲低PDIA3P1则诱导了相反的作用。进一步的研究表明,PDIA3P1可作为ceRNA发挥功能,使miR-124-3p海绵化以调节RELA表达并激活下游NF-κB途径,从而促进神经胶质瘤细胞的MES转化。另外,证实缺氧诱导因子1直接结合至PDIA3P1启动子区域并激活其转录。总之,PDIA3P1是缺氧和神经胶质瘤通过PDIA3P1-miR-124-3p-RELA轴进行MES转换之间的关键环节,它可能是神经胶质瘤治疗的预后指标和潜在治疗靶标。体外研究表明,PDIA3P1的过表达增强了胶质瘤细胞的迁移和侵袭能力,而敲低PDIA3P1则诱导了相反的作用。进一步的研究表明,PDIA3P1可作为ceRNA发挥功能,使miR-124-3p海绵化以调节RELA表达并激活下游NF-κB途径,从而促进神经胶质瘤细胞的MES转化。另外,证实了缺氧诱导因子1直接结合至PDIA3P1启动子区域并激活其转录。总之,PDIA3P1是缺氧和神经胶质瘤通过PDIA3P1-miR-124-3p-RELA轴进行MES转换之间的关键环节,它可能是神经胶质瘤治疗的预后指标和潜在治疗靶标。体外研究表明,PDIA3P1的过表达增强了胶质瘤细胞的迁移和侵袭能力,而敲低PDIA3P1则诱导了相反的作用。进一步的研究表明,PDIA3P1可作为ceRNA发挥功能,使miR-124-3p海绵化以调节RELA表达并激活下游NF-κB途径,从而促进神经胶质瘤细胞的MES转化。另外,证实了缺氧诱导因子1直接结合至PDIA3P1启动子区域并激活其转录。总之,PDIA3P1是缺氧和神经胶质瘤通过PDIA3P1-miR-124-3p-RELA轴进行MES转换之间的关键环节,它可能是神经胶质瘤治疗的预后指标和潜在治疗靶标。进一步的研究表明,PDIA3P1可作为ceRNA发挥功能,使miR-124-3p充满海绵,从而调节RELA表达并激活下游NF-κB途径,从而促进神经胶质瘤细胞的MES转化。另外,证实了缺氧诱导因子1直接结合至PDIA3P1启动子区域并激活其转录。总之,PDIA3P1是缺氧和神经胶质瘤通过PDIA3P1-miR-124-3p-RELA轴进行MES转换之间的关键环节,它可能是神经胶质瘤治疗的预后指标和潜在治疗靶标。进一步的研究表明,PDIA3P1可作为ceRNA发挥功能,使miR-124-3p海绵化以调节RELA表达并激活下游NF-κB途径,从而促进神经胶质瘤细胞的MES转化。另外,证实了缺氧诱导因子1直接结合至PDIA3P1启动子区域并激活其转录。总之,PDIA3P1是缺氧和神经胶质瘤通过PDIA3P1-miR-124-3p-RELA轴进行MES转换之间的关键环节,它可能是神经胶质瘤治疗的预后指标和潜在治疗靶标。
更新日期:2020-03-03
中文翻译:

缺氧诱导的lncRNA PDIA3P1通过胶质瘤中miR-124-3p的海绵化促进间质转化。
缺氧是神经胶质瘤恶性进展的关键因素,尤其是对于高度侵入性的间充质(MES)亚型。但是在缺氧诱导的神经胶质瘤MES转移的详细机制仍不清楚。伪基因曾经被认为是进化的非功能性遗迹,正在成为人类肿瘤发生和发展的关键因素。在这里,我们调查了缺氧诱导的神经胶质瘤MES转换中蛋白质二硫键异构酶家族A成员3假基因1(PDIA3P1)的临床意义,生物学功能和机制。在这项研究中,我们发现PDIA3P1的表达与神经胶质瘤患者的肿瘤程度,转录组亚型和预后密切相关。富集分析发现PDIA3P1高表达与上皮-间质转化,细胞外基质(ECM)拆卸,和血管生成。体外研究表明,PDIA3P1的过表达增强了胶质瘤细胞的迁移和侵袭能力,而敲低PDIA3P1则诱导了相反的作用。进一步的研究表明,PDIA3P1可作为ceRNA发挥功能,使miR-124-3p海绵化以调节RELA表达并激活下游NF-κB途径,从而促进神经胶质瘤细胞的MES转化。另外,证实缺氧诱导因子1直接结合至PDIA3P1启动子区域并激活其转录。总之,PDIA3P1是缺氧和神经胶质瘤通过PDIA3P1-miR-124-3p-RELA轴进行MES转换之间的关键环节,它可能是神经胶质瘤治疗的预后指标和潜在治疗靶标。体外研究表明,PDIA3P1的过表达增强了胶质瘤细胞的迁移和侵袭能力,而敲低PDIA3P1则诱导了相反的作用。进一步的研究表明,PDIA3P1可作为ceRNA发挥功能,使miR-124-3p海绵化以调节RELA表达并激活下游NF-κB途径,从而促进神经胶质瘤细胞的MES转化。另外,证实了缺氧诱导因子1直接结合至PDIA3P1启动子区域并激活其转录。总之,PDIA3P1是缺氧和神经胶质瘤通过PDIA3P1-miR-124-3p-RELA轴进行MES转换之间的关键环节,它可能是神经胶质瘤治疗的预后指标和潜在治疗靶标。体外研究表明,PDIA3P1的过表达增强了胶质瘤细胞的迁移和侵袭能力,而敲低PDIA3P1则诱导了相反的作用。进一步的研究表明,PDIA3P1可作为ceRNA发挥功能,使miR-124-3p海绵化以调节RELA表达并激活下游NF-κB途径,从而促进神经胶质瘤细胞的MES转化。另外,证实了缺氧诱导因子1直接结合至PDIA3P1启动子区域并激活其转录。总之,PDIA3P1是缺氧和神经胶质瘤通过PDIA3P1-miR-124-3p-RELA轴进行MES转换之间的关键环节,它可能是神经胶质瘤治疗的预后指标和潜在治疗靶标。进一步的研究表明,PDIA3P1可作为ceRNA发挥功能,使miR-124-3p充满海绵,从而调节RELA表达并激活下游NF-κB途径,从而促进神经胶质瘤细胞的MES转化。另外,证实了缺氧诱导因子1直接结合至PDIA3P1启动子区域并激活其转录。总之,PDIA3P1是缺氧和神经胶质瘤通过PDIA3P1-miR-124-3p-RELA轴进行MES转换之间的关键环节,它可能是神经胶质瘤治疗的预后指标和潜在治疗靶标。进一步的研究表明,PDIA3P1可作为ceRNA发挥功能,使miR-124-3p海绵化以调节RELA表达并激活下游NF-κB途径,从而促进神经胶质瘤细胞的MES转化。另外,证实了缺氧诱导因子1直接结合至PDIA3P1启动子区域并激活其转录。总之,PDIA3P1是缺氧和神经胶质瘤通过PDIA3P1-miR-124-3p-RELA轴进行MES转换之间的关键环节,它可能是神经胶质瘤治疗的预后指标和潜在治疗靶标。



























 京公网安备 11010802027423号
京公网安备 11010802027423号