Coordination Chemistry Reviews ( IF 20.6 ) Pub Date : 2020-03-02 , DOI: 10.1016/j.ccr.2020.213253 Jaime M. Murphy , Brian A. Powell , Julia L. Brumaghim
|
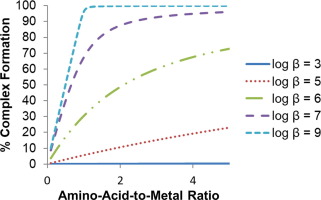
Metal ion interactions with weakly coordinating ligands, such as amino acids, are dependent on several factors, including metal ion availability, metal ion propensity for hydrolysis, ligand availability, and thermodynamic stability, as measured by stability constants. Metal ions in biological systems are often controlled by highly specific chaperone, transport, and storage proteins. Disruption in the homeostasis of redox active metal ions, such as Cu(I), Cu(II), Fe(II), and Fe(III), has been linked to increased oxidative damage and disease. Weakly binding ligands such as amino acids may play an active role in mitigating this metal-mediated damage, but a comprehensive understanding of the availability and thermodynamic likelihood of coordination must be understood to accurately predict complex formation in a competitive environment. This review presents an overview of amino acid stability constants with Cu(I), Cu(II), Fe(II), and Fe(III), the most common redox-active metal ions in biological systems. Specific attention is given to sulfur- and selenium-containing amino acids, since their interactions with Cu(I) and Fe(II) is of particular biological interest. This review also describes methods available for stability constant determination, with particular attention to specific difficulties encountered when working with weakly binding ligands and each of these four metal ions. Finally, the potential biological implications of these results are discussed based on reported stability constants as well as amino acid, copper, and iron bioavailability.
中文翻译:

具有氨基酸的生物相关氧化还原活性金属的稳定性常数:弱结合配体的挑战
金属离子与配位能力较弱的配体(例如氨基酸)的相互作用取决于几个因素,包括金属离子的利用度,金属离子的水解倾向,配体的利用度和热力学稳定性(以稳定性常数衡量)。生物系统中的金属离子通常由高度特异性的伴侣蛋白,转运蛋白和储存蛋白控制。氧化还原活性金属离子(例如Cu(I),Cu(II),Fe(II)和Fe(III))的体内平衡破坏与增加的氧化损伤和疾病有关。弱结合的配体(例如氨基酸)可能在减轻这种金属介导的损害中起积极作用,但是必须理解对配位的有效性和热力学可能性的全面理解,才能准确预测竞争环境中的复合物形成。这篇综述介绍了氨基酸(Cu),铜(II),铁(II)和铁(III)的氨基酸稳定常数,这是生物系统中最常见的氧化还原活性金属离子。由于含硫和硒的氨基酸与Cu(I)和Fe(II)的相互作用具有特殊的生物学意义,因此应特别注意。这篇综述还描述了可用于稳定性常数测定的方法,尤其要注意使用弱结合配体和这四种金属离子中的每一种时遇到的特定困难。最后,基于报告的稳定性常数以及氨基酸,铜和铁的生物利用度,讨论了这些结果的潜在生物学意义。生物系统中最常见的氧化还原活性金属离子。由于含硫和硒的氨基酸与Cu(I)和Fe(II)的相互作用具有特殊的生物学意义,因此应特别注意。这篇综述还描述了可用于稳定性常数测定的方法,尤其要注意使用弱结合配体和这四种金属离子中的每一种时遇到的特定困难。最后,基于报告的稳定性常数以及氨基酸,铜和铁的生物利用度,讨论了这些结果的潜在生物学意义。生物系统中最常见的氧化还原活性金属离子。由于含硫和硒的氨基酸与Cu(I)和Fe(II)的相互作用具有特殊的生物学意义,因此应特别注意。这篇综述还描述了可用于稳定常数测定的方法,尤其要注意使用弱结合配体和这四种金属离子中的每一种时遇到的特定困难。最后,基于报告的稳定性常数以及氨基酸,铜和铁的生物利用度,讨论了这些结果的潜在生物学含义。这篇综述还描述了可用于稳定性常数测定的方法,尤其要注意使用弱结合配体和这四种金属离子中的每一种时遇到的特定困难。最后,基于报告的稳定性常数以及氨基酸,铜和铁的生物利用度,讨论了这些结果的潜在生物学意义。这篇综述还描述了可用于稳定常数测定的方法,尤其要注意使用弱结合配体和这四种金属离子中的每一种时遇到的特定困难。最后,基于报告的稳定性常数以及氨基酸,铜和铁的生物利用度,讨论了这些结果的潜在生物学含义。


























 京公网安备 11010802027423号
京公网安备 11010802027423号