Molecular Cell ( IF 16.0 ) Pub Date : 2020-03-03 , DOI: 10.1016/j.molcel.2020.02.001 Wei He 1 , H B D Prasada Rao 1 , Shangming Tang 1 , Nikhil Bhagwat 1 , Dhananjaya S Kulkarni 1 , Yunmei Ma 1 , Maria A W Chang 2 , Christie Hall 2 , Junxi Wang Bragg 2 , Harrison S Manasca 2 , Christa Baker 2 , Gerrik F Verhees 2 , Lepakshi Ranjha 3 , Xiangyu Chen 4 , Nancy M Hollingsworth 4 , Petr Cejka 3 , Neil Hunter 5
|
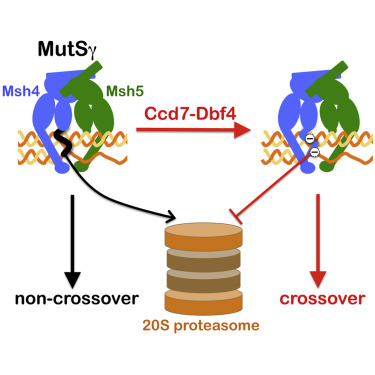
Crossover recombination is essential for accurate chromosome segregation during meiosis. The MutSγ complex, Msh4-Msh5, facilitates crossing over by binding and stabilizing nascent recombination intermediates. We show that these activities are governed by regulated proteolysis. MutSγ is initially inactive for crossing over due to an N-terminal degron on Msh4 that renders it unstable by directly targeting proteasomal degradation. Activation of MutSγ requires the Dbf4-dependent kinase Cdc7 (DDK), which directly phosphorylates and thereby neutralizes the Msh4 degron. Genetic requirements for Msh4 phosphorylation indicate that DDK targets MutSγ only after it has bound to nascent joint molecules (JMs) in the context of synapsing chromosomes. Overexpression studies confirm that the steady-state level of Msh4, not phosphorylation per se, is the critical determinant for crossing over. At the DNA level, Msh4 phosphorylation enables the formation and crossover-biased resolution of double-Holliday Junction intermediates. Our study establishes regulated protein degradation as a fundamental mechanism underlying meiotic crossing over.
中文翻译:

MutSγ的调控的蛋白水解控制减数分裂交叉。
交叉重组对于减数分裂过程中准确的染色体分离至关重要。MutSγ复合物Msh4-Msh5通过结合和稳定新生重组中间体来促进交叉。我们表明这些活动受规管的蛋白水解作用。MutSγ最初由于Msh4上的一个N末端degron而无法跨越,因此通过直接靶向蛋白酶体降解使其变得不稳定。MutSγ的激活需要Dbf4依赖性激酶Cdc7(DDK),后者直接磷酸化,从而中和Msh4 degron。Msh4磷酸化的遗传要求表明,DDK仅在突变的染色体中结合到新生关节分子(JM)后才靶向MutSγ。过度表达研究证实,Msh4处于稳态水平,而不是磷酸化本身,是越界的关键决定因素。在DNA水平上,Msh4磷酸化使双Holliday Junction中间体的形成和交叉偏向拆分成为可能。我们的研究建立了调节蛋白质降解作为减数分裂交叉的基本机制。



























 京公网安备 11010802027423号
京公网安备 11010802027423号