当前位置:
X-MOL 学术
›
Colloids Surf. B Biointerfaces
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Performance of oil-in-water emulsions stabilized by different types of surface-active components.
Colloids and Surfaces B: Biointerfaces ( IF 5.8 ) Pub Date : 2020-03-03 , DOI: 10.1016/j.colsurfb.2020.110939 Bruna Barbon Paulo 1 , Izabela Dutra Alvim 2 , Gary Reineccius 3 , Ana Silvia Prata 1
Colloids and Surfaces B: Biointerfaces ( IF 5.8 ) Pub Date : 2020-03-03 , DOI: 10.1016/j.colsurfb.2020.110939 Bruna Barbon Paulo 1 , Izabela Dutra Alvim 2 , Gary Reineccius 3 , Ana Silvia Prata 1
Affiliation
|
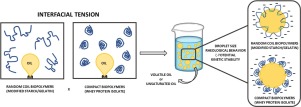
The emulsion stability depends on the physicochemical properties of the dispersed phase and their interaction with the continuous phase. Surface-active compounds (SAC) are added in emulsions to reduce the interfacial tension (IT) between these phases and keep the oil droplets stabilized. Moreover, small amounts of SAC can occupy intermolecular voids in the dried matrix, reducing the oxidation. However, the formulation must reflect a trade-off between protection and emulsion stabilization. Therefore, this work aimed to identify the minimum concentration of SAC (modified starch-MS, gelatin-GE, and whey protein isolate-WPI) ranging from 0.48 to 6 % (w/w) to form and stabilize droplets of an unsaturated triglyceride (fish oil-FO) or a volatile oil (orange essential oil-OEO). GE did not change the IT (6.7 mN/m) and stabilized the emulsions only through an increase of the viscosity (∼42 mPas for FO-emulsions and ∼97 mPas for OEO-emulsions), presenting high droplet size (∼10 μm) and low surface charge (∼1.5 mV). WPI reduced the IT to a limit value (4.5 mN/m at 1.2 % w/w for OEO and 5.3 mN/m at 2.4 % w/w for FO), whereas MS reduce constantly the IT with the increase of the concentration for both oils (∼4.2 mN/m at 6 % w/w). Both WPI and MS-emulsions presented similar droplet size (∼2.0 μm), but WPI presented higher surface charge of WPI-emulsions (-45 mV) than MS-emulsions (-30 mV). This study allowed to gain a consistent understanding of structure-property relationships on the use of SAC in emulsions.
中文翻译:

不同类型的表面活性组分可稳定水包油型乳液的性能。
乳液的稳定性取决于分散相的物理化学性质及其与连续相的相互作用。在乳液中添加表面活性化合物(SAC),以降低这些相之间的界面张力(IT),并使油滴保持稳定。而且,少量的SAC可以占据干燥基质中的分子间空隙,从而减少氧化。但是,该配方必须反映出保护和乳液稳定之间的权衡。因此,这项工作旨在确定SAC(改性淀粉-MS,明胶-GE和乳清蛋白分离物-WPI)的最低浓度为0.48%至6%(w / w),以形成和稳定不饱和甘油三酸酯的液滴(鱼油-FO)或挥发油(橙色精油-OEO)。GE并未更改IT(6。7 mN / m)并仅通过增加粘度来稳定乳液(FO乳液约为42 mPas,而OEO乳液约为97 mPas),具有高液滴尺寸(〜10μm)和低表面电荷(〜1.5) mV)。WPI将IT降低到极限值(OEO为1.2%w / w时为4.5 mN / m,FO为2.4%w / w时为5.3 mN / m,对于FO),而MS则随着两种浓度的增加而不断降低IT油(约4.2 mN / m,6%w / w)。WPI和MS乳液的液滴大小相似(约2.0μm),但WPI的WPI乳液(-45 mV)的表面电荷高于MS乳液(-30 mV)。这项研究使人们对乳液中使用SAC的结构-性质关系有了一致的了解。具有高液滴尺寸(〜10μm)和低表面电荷(〜1.5 mV)。WPI将IT降低到极限值(OEO为1.2%w / w时为4.5 mN / m,FO为2.4%w / w时为5.3 mN / m,对于FO),而MS则随着两种浓度的增加而不断降低IT油(约4.2 mN / m,6%w / w)。WPI和MS乳液的液滴大小相似(约2.0μm),但WPI的WPI乳液(-45 mV)的表面电荷高于MS乳液(-30 mV)。这项研究使人们对乳液中使用SAC的结构-性质关系有了一致的了解。具有高液滴尺寸(〜10μm)和低表面电荷(〜1.5 mV)。WPI将IT降低到一个极限值(OEO为1.2%w / w时为4.5 mN / m,FO为2.4%w / w时为5.3 mN / m,对于FO),而MS则随着浓度的增加而不断降低IT油(约4.2 mN / m,6%w / w)。WPI和MS乳液的液滴大小相似(约2.0μm),但WPI的WPI乳液(-45 mV)的表面电荷高于MS乳液(-30 mV)。这项研究使人们对乳液中使用SAC的结构-性质关系有了一致的了解。0μm),但是WPI呈现的WPI乳液(-45 mV)的表面电荷高于MS乳液(-30 mV)。这项研究使人们对乳液中使用SAC的结构-性质关系有了一致的了解。0μm),但是WPI呈现的WPI乳液(-45 mV)的表面电荷高于MS乳液(-30 mV)。这项研究使人们对乳液中使用SAC的结构-性质关系有了一致的了解。
更新日期:2020-03-03
中文翻译:

不同类型的表面活性组分可稳定水包油型乳液的性能。
乳液的稳定性取决于分散相的物理化学性质及其与连续相的相互作用。在乳液中添加表面活性化合物(SAC),以降低这些相之间的界面张力(IT),并使油滴保持稳定。而且,少量的SAC可以占据干燥基质中的分子间空隙,从而减少氧化。但是,该配方必须反映出保护和乳液稳定之间的权衡。因此,这项工作旨在确定SAC(改性淀粉-MS,明胶-GE和乳清蛋白分离物-WPI)的最低浓度为0.48%至6%(w / w),以形成和稳定不饱和甘油三酸酯的液滴(鱼油-FO)或挥发油(橙色精油-OEO)。GE并未更改IT(6。7 mN / m)并仅通过增加粘度来稳定乳液(FO乳液约为42 mPas,而OEO乳液约为97 mPas),具有高液滴尺寸(〜10μm)和低表面电荷(〜1.5) mV)。WPI将IT降低到极限值(OEO为1.2%w / w时为4.5 mN / m,FO为2.4%w / w时为5.3 mN / m,对于FO),而MS则随着两种浓度的增加而不断降低IT油(约4.2 mN / m,6%w / w)。WPI和MS乳液的液滴大小相似(约2.0μm),但WPI的WPI乳液(-45 mV)的表面电荷高于MS乳液(-30 mV)。这项研究使人们对乳液中使用SAC的结构-性质关系有了一致的了解。具有高液滴尺寸(〜10μm)和低表面电荷(〜1.5 mV)。WPI将IT降低到极限值(OEO为1.2%w / w时为4.5 mN / m,FO为2.4%w / w时为5.3 mN / m,对于FO),而MS则随着两种浓度的增加而不断降低IT油(约4.2 mN / m,6%w / w)。WPI和MS乳液的液滴大小相似(约2.0μm),但WPI的WPI乳液(-45 mV)的表面电荷高于MS乳液(-30 mV)。这项研究使人们对乳液中使用SAC的结构-性质关系有了一致的了解。具有高液滴尺寸(〜10μm)和低表面电荷(〜1.5 mV)。WPI将IT降低到一个极限值(OEO为1.2%w / w时为4.5 mN / m,FO为2.4%w / w时为5.3 mN / m,对于FO),而MS则随着浓度的增加而不断降低IT油(约4.2 mN / m,6%w / w)。WPI和MS乳液的液滴大小相似(约2.0μm),但WPI的WPI乳液(-45 mV)的表面电荷高于MS乳液(-30 mV)。这项研究使人们对乳液中使用SAC的结构-性质关系有了一致的了解。0μm),但是WPI呈现的WPI乳液(-45 mV)的表面电荷高于MS乳液(-30 mV)。这项研究使人们对乳液中使用SAC的结构-性质关系有了一致的了解。0μm),但是WPI呈现的WPI乳液(-45 mV)的表面电荷高于MS乳液(-30 mV)。这项研究使人们对乳液中使用SAC的结构-性质关系有了一致的了解。


























 京公网安备 11010802027423号
京公网安备 11010802027423号