当前位置:
X-MOL 学术
›
Chem. Geol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Natural organic matter decreases uptake of W(VI), and reduces W(VI) to W(V), during adsorption to ferrihydrite
Chemical Geology ( IF 3.9 ) Pub Date : 2020-05-01 , DOI: 10.1016/j.chemgeo.2020.119567 Huihui Du , Zelin Xu , Meng Hu , Huanjing Zhang , Caroline L. Peacock , Xin Liu , Ning Nie , Qin Xue , Ming Lei , Boqing Tie
Chemical Geology ( IF 3.9 ) Pub Date : 2020-05-01 , DOI: 10.1016/j.chemgeo.2020.119567 Huihui Du , Zelin Xu , Meng Hu , Huanjing Zhang , Caroline L. Peacock , Xin Liu , Ning Nie , Qin Xue , Ming Lei , Boqing Tie
|
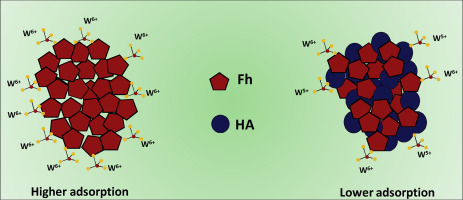
Abstract Tungsten is both naturally occurring and an anthropogenically released contaminant metal in soils, sediments and water systems that typically exits as the soluble tungstate oxyanions, W(VI)O42−. Tungsten mobility and fate are strongly dependent on the adsorption of tungstate to mineral surfaces. However, environmental mineral surfaces are commonly coated with natural organic matter (NOM), and the role of this coating in the tungsten adsorption process, and thus in controlling tungsten reactivity and transport, is unclear. This study investigates W(VI) adsorption to ferrihydrite (Fh), a ubiquitous iron (hydr)oxide in soils and sediments, both in the absence and presence of humic acid (HA), a widely occurring type of NOM, using batch experiments coupled with spectroscopic and thermodynamic techniques. Kinetic results indicate that access to the adsorption sites for W(VI) on the organomineral surfaces is limited when Fh is coprecipitated with HA. Commensurate with this observation, batch experiments show that HA decreases W(VI) adsorption to Fh over a wide pH range (4–11), and this inhibitory effect is more pronounced at higher HA concentration. X-ray photoelectron spectroscopy (XPS) measurements demonstrate the formation of inner-sphere type W complexes on both the Fh and HA fraction of the Fh-HA binary composite. In particular, ~40% of the adsorbed W(VI) species is reduced to W(V) in the presence of HA. Attenuated total reflectance Fourier transform infrared spectroscopy (ATR-FTIR) results show the presence of poly tungstate species on Fh, particularly at lower pH and in the presence of HA. Isothermal titration calorimetry shows that W(VI) adsorption to Fh is an exothermic process both in the presence and absence of HA, and that process is accompanied by a positive entropy. The findings of this work suggest that NOM not only mobilizes tungstate but also reduces tungstate from W(VI) to W(V) at environmental iron (hydr)oxide-water interfaces, which is of significance for evaluating the migration and bioavailability of tungsten in both natural and contaminated environments.
中文翻译:

在吸附到水铁矿的过程中,天然有机物减少了 W(VI) 的吸收,并将 W(VI) 还原为 W(V)
摘要 钨是土壤、沉积物和水系统中天然存在的和人为释放的污染物金属,通常以可溶性钨酸氧阴离子 W(VI)O42- 形式存在。钨的迁移率和命运很大程度上取决于钨酸盐对矿物表面的吸附。然而,环境矿物表面通常涂有天然有机物(NOM),这种涂层在钨吸附过程中的作用,从而控制钨的反应性和运输,尚不清楚。本研究使用分批实验研究 W(VI) 对水铁矿 (Fh) 的吸附,水铁矿 (Fh) 是土壤和沉积物中普遍存在的氧化铁(氢)氧化物,在腐植酸 (HA)(一种广泛存在的 NOM 类型)不存在和存在下用光谱和热力学技术。动力学结果表明,当 Fh 与 HA 共沉淀时,有机矿物表面上 W(VI) 的吸附位点受到限制。与这一观察结果相称,批量实验表明,HA 在较宽的 pH 范围(4-11)内降低了 W(VI) 对 Fh 的吸附,并且这种抑制作用在 HA 浓度较高时更为明显。X 射线光电子能谱 (XPS) 测量表明在 Fh-HA 二元复合材料的 Fh 和 HA 部分上形成了内球型 W 复合物。特别是,在 HA 存在下,约 40% 的吸附 W(VI) 物质被还原为 W(V)。衰减全反射傅里叶变换红外光谱 (ATR-FTIR) 结果显示 Fh 上存在聚钨酸盐物质,尤其是在较低的 pH 值和存在 HA 的情况下。等温滴定量热法表明 W(VI) 吸附到 Fh 是在存在和不存在 HA 的情况下的放热过程,并且该过程伴随着正熵。这项工作的结果表明,NOM 不仅可以动员钨酸盐,而且还可以将环境铁(氢)氧化物-水界面处的钨酸盐从 W(VI) 还原为 W(V),这对于评估钨在环境中的迁移和生物利用度具有重要意义。自然环境和污染环境。
更新日期:2020-05-01
中文翻译:

在吸附到水铁矿的过程中,天然有机物减少了 W(VI) 的吸收,并将 W(VI) 还原为 W(V)
摘要 钨是土壤、沉积物和水系统中天然存在的和人为释放的污染物金属,通常以可溶性钨酸氧阴离子 W(VI)O42- 形式存在。钨的迁移率和命运很大程度上取决于钨酸盐对矿物表面的吸附。然而,环境矿物表面通常涂有天然有机物(NOM),这种涂层在钨吸附过程中的作用,从而控制钨的反应性和运输,尚不清楚。本研究使用分批实验研究 W(VI) 对水铁矿 (Fh) 的吸附,水铁矿 (Fh) 是土壤和沉积物中普遍存在的氧化铁(氢)氧化物,在腐植酸 (HA)(一种广泛存在的 NOM 类型)不存在和存在下用光谱和热力学技术。动力学结果表明,当 Fh 与 HA 共沉淀时,有机矿物表面上 W(VI) 的吸附位点受到限制。与这一观察结果相称,批量实验表明,HA 在较宽的 pH 范围(4-11)内降低了 W(VI) 对 Fh 的吸附,并且这种抑制作用在 HA 浓度较高时更为明显。X 射线光电子能谱 (XPS) 测量表明在 Fh-HA 二元复合材料的 Fh 和 HA 部分上形成了内球型 W 复合物。特别是,在 HA 存在下,约 40% 的吸附 W(VI) 物质被还原为 W(V)。衰减全反射傅里叶变换红外光谱 (ATR-FTIR) 结果显示 Fh 上存在聚钨酸盐物质,尤其是在较低的 pH 值和存在 HA 的情况下。等温滴定量热法表明 W(VI) 吸附到 Fh 是在存在和不存在 HA 的情况下的放热过程,并且该过程伴随着正熵。这项工作的结果表明,NOM 不仅可以动员钨酸盐,而且还可以将环境铁(氢)氧化物-水界面处的钨酸盐从 W(VI) 还原为 W(V),这对于评估钨在环境中的迁移和生物利用度具有重要意义。自然环境和污染环境。


























 京公网安备 11010802027423号
京公网安备 11010802027423号