Biochimica et Biophysica Acta (BBA) - Molecular Basis of Disease ( IF 6.2 ) Pub Date : 2020-03-03 , DOI: 10.1016/j.bbadis.2020.165754 Ajit C. Dhadve , Kishore Hari , Bharat Rekhi , Mohit Kumar Jolly , Abhijit De , Pritha Ray
|
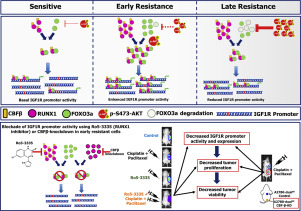
Hyperactive Insulin like growth factor-1-receptor (IGF1R) signalling is associated with development of therapy resistance in many cancers. We recently reported a pulsatile nature of IGF1R during acquirement of platinum-taxol resistance in Epithelial Ovarian Cancer (EOC) cells and a therapy induced upregulation in IGF1R expression in tumors of a small cohort of high grade serous EOC patients. Here, we report Runt-related transcription factor 1 (RUNX1) as a novel transcriptional regulator which along with another known regulator Forkhead Box O3 (FOXO3a), drives the dynamic modulation of IGF1R expression during platinum-taxol resistance development in EOC cells. RUNX1-FOXO3a cooperatively bind to IGF1R promoter and produce a transcriptional surge during onset of resistance and such co-operativity falls apart when cells attain maximal resistance resulting in decreased IGF1R expression. The intriguing descending trend in IGF1R and FOXO3a expressions is caused by a Protein Kinase B (AKT)-FOXO3a negative feedback loop exclusively present in the highly resistant cells eliciting the pulsatile behaviour of IGF1R and FOXO3a. In vivo molecular imaging revealed that RUNX1 inhibition causes significant attenuation of the IGF1R promoter activity, decreased tumorigenicity and enhanced drug sensitivity of tumors of early resistant cells. Altogether our findings delineate a dynamic interplay between several molecular regulators driving pulsatile IGF1R expression and identify a new avenue for targeting EOC through RUNX1-IGF1R axis during acquirement of chemoresistance.
中文翻译:

在获得化学抗性过程中,对脉冲性IGF1R表达基础的RUNX1和FOXO3a之间的分子相互作用进行解码
在许多癌症中,像生长因子-1-受体(IGF1R)信号一样活跃的胰岛素与治疗抗性的发展有关。我们最近报道了在上皮性卵巢癌(EOC)细胞中铂-紫杉醇耐药性获得过程中IGF1R的搏动性,该疗法在少量高级别浆液性EOC患者队列的肿瘤中诱导了IGF1R表达的上调。在这里,我们报告矮子相关的转录因子1(RUNX1)作为一种新型的转录调节因子,与另一种已知的调节器Forkhead Box O3(FOXO3a)一起,在EOC细胞的铂-紫杉醇抗性发展过程中驱动IGF1R表达的动态调节。RUNX1-FOXO3a与IGF1R启动子协同结合并在抗药性发作期间产生转录激增,当细胞获得最大抗药性导致IGF1R表达降低时,这种协同作用就会瓦解。IGF1R和FOXO3a表达的有趣下降趋势是由蛋白激酶B(AKT)-FOXO3a负反馈回路引起的,该负反馈回路仅存在于引起IGF1R和FOXO3a搏动行为的高抗性细胞中。体内分子成像显示,RUNX1抑制作用会导致IGF1R启动子活性显着减弱,致瘤性降低,早期耐药细胞肿瘤的药物敏感性增强。总的来说,我们的发现描述了驱动脉动性IGF1R表达的几种分子调节剂之间的动态相互作用,并确定了在化学耐药性获得过程中通过RUNX1-IGF1R轴靶向EOC的新途径。


























 京公网安备 11010802027423号
京公网安备 11010802027423号