当前位置:
X-MOL 学术
›
J. Hepatol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Inhibition of PU.1 ameliorates metabolic dysfunction and non-alcoholic steatohepatitis
Journal of Hepatology ( IF 25.7 ) Pub Date : 2020-08-01 , DOI: 10.1016/j.jhep.2020.02.025 Qiongming Liu 1 , Junjie Yu 2 , Liheng Wang 2 , Yuliang Tang 3 , Quan Zhou 4 , Shuhui Ji 4 , Yi Wang 5 , Luis Santos 6 , Rebecca A Haeusler 7 , Jianwen Que 8 , Prashant Rajbhandari 6 , Xiaoguang Lei 3 , Luca Valenti 9 , Utpal B Pajvani 2 , Jun Qin 10 , Li Qiang 7
Journal of Hepatology ( IF 25.7 ) Pub Date : 2020-08-01 , DOI: 10.1016/j.jhep.2020.02.025 Qiongming Liu 1 , Junjie Yu 2 , Liheng Wang 2 , Yuliang Tang 3 , Quan Zhou 4 , Shuhui Ji 4 , Yi Wang 5 , Luis Santos 6 , Rebecca A Haeusler 7 , Jianwen Que 8 , Prashant Rajbhandari 6 , Xiaoguang Lei 3 , Luca Valenti 9 , Utpal B Pajvani 2 , Jun Qin 10 , Li Qiang 7
Affiliation
|
BACKGROUND & AIMS
Obesity is a well-established risk factor for type 2 diabetes (T2D) and nonalcoholic steatohepatitis (NASH), but the underlying mechanisms remain incompletely understood. Here, we aimed to identify novel pathogenic factor and therapeutic target of liver metabolic dysfunctions. METHODS
We applied tandem quantitative proteomics strategy to enrich and identify transcription factors (TFs) induced in the obese liver. We used flow cytometry of liver cells to analyze the source of the induced TF. We employed conditional knockout mice, shRNA, and small-molecule inhibitors to test metabolic consequences of the induction of identified TF. Finally, we validated mouse data in patient liver biopsies. RESULTS
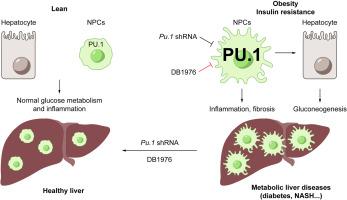
We identified PU.1/SPI1, the master hematopoietic regulator, as one of the most up-regulated TFs in livers from diet-induced (DIO) and genetically obese (db/db) mice. Targeting PU.1 in the whole liver-but not hepatocytes alone-significantly improved glucose homeostasis and suppressed liver inflammation. Consistently, treatment with the PU.1 inhibitor DB1976 markedly reduced inflammation and improved glucose homeostasis and dyslipidemia in DIO mice, and strongly suppressed glucose intolerance, liver steatosis, inflammation, and fibrosis in a dietary NASH mouse model. Furthermore, hepatic PU.1 expression was positively correlated with insulin resistance and inflammation in liver biopsies from patients. CONCLUSIONS
These data suggest that the elevated hematopoietic factor PU.1 promotes liver metabolic dysfunctions, and may be a useful therapeutic target for obesity, insulin resistance/T2D, and NASH.
中文翻译:

抑制 PU.1 可改善代谢功能障碍和非酒精性脂肪性肝炎
背景和目的 肥胖是 2 型糖尿病 (T2D) 和非酒精性脂肪性肝炎 (NASH) 的公认危险因素,但其潜在机制仍未完全了解。在这里,我们旨在确定肝脏代谢功能障碍的新致病因素和治疗靶点。方法我们应用串联定量蛋白质组学策略来富集和鉴定在肥胖肝脏中诱导的转录因子 (TF)。我们使用肝细胞的流式细胞术来分析诱导 TF 的来源。我们采用条件敲除小鼠、shRNA 和小分子抑制剂来测试诱导已识别 TF 的代谢后果。最后,我们验证了患者肝脏活检中的小鼠数据。结果我们确定了 PU.1/SPI1,主要的造血调节剂,作为饮食诱导 (DIO) 和遗传性肥胖 (db/db) 小鼠肝脏中最上调的 TF 之一。在整个肝脏中靶向 PU.1 - 但不是单独的肝细胞 - 显着改善了葡萄糖稳态并抑制了肝脏炎症。一致地,用 PU.1 抑制剂 DB1976 治疗显着减少了 DIO 小鼠的炎症并改善了葡萄糖稳态和血脂异常,并强烈抑制了饮食 NASH 小鼠模型中的葡萄糖耐受不良、肝脂肪变性、炎症和纤维化。此外,肝脏 PU.1 表达与患者肝脏活检中的胰岛素抵抗和炎症呈正相关。结论 这些数据表明,升高的造血因子 PU.1 会促进肝脏代谢功能障碍,并且可能是肥胖、胰岛素抵抗/T2D 和 NASH 的有用治疗靶点。
更新日期:2020-08-01
中文翻译:

抑制 PU.1 可改善代谢功能障碍和非酒精性脂肪性肝炎
背景和目的 肥胖是 2 型糖尿病 (T2D) 和非酒精性脂肪性肝炎 (NASH) 的公认危险因素,但其潜在机制仍未完全了解。在这里,我们旨在确定肝脏代谢功能障碍的新致病因素和治疗靶点。方法我们应用串联定量蛋白质组学策略来富集和鉴定在肥胖肝脏中诱导的转录因子 (TF)。我们使用肝细胞的流式细胞术来分析诱导 TF 的来源。我们采用条件敲除小鼠、shRNA 和小分子抑制剂来测试诱导已识别 TF 的代谢后果。最后,我们验证了患者肝脏活检中的小鼠数据。结果我们确定了 PU.1/SPI1,主要的造血调节剂,作为饮食诱导 (DIO) 和遗传性肥胖 (db/db) 小鼠肝脏中最上调的 TF 之一。在整个肝脏中靶向 PU.1 - 但不是单独的肝细胞 - 显着改善了葡萄糖稳态并抑制了肝脏炎症。一致地,用 PU.1 抑制剂 DB1976 治疗显着减少了 DIO 小鼠的炎症并改善了葡萄糖稳态和血脂异常,并强烈抑制了饮食 NASH 小鼠模型中的葡萄糖耐受不良、肝脂肪变性、炎症和纤维化。此外,肝脏 PU.1 表达与患者肝脏活检中的胰岛素抵抗和炎症呈正相关。结论 这些数据表明,升高的造血因子 PU.1 会促进肝脏代谢功能障碍,并且可能是肥胖、胰岛素抵抗/T2D 和 NASH 的有用治疗靶点。


























 京公网安备 11010802027423号
京公网安备 11010802027423号