当前位置:
X-MOL 学术
›
Chem. Res. Toxicol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Surface Functionalization of Pegylated Gold Nanoparticles with Antioxidants Suppresses Nanoparticle-Induced Oxidative Stress and Neurotoxicity.
Chemical Research in Toxicology ( IF 4.1 ) Pub Date : 2020-03-03 , DOI: 10.1021/acs.chemrestox.9b00368 Xiaojie Zhang 1 , Xueling Guo 1 , Xiaoxuan Kang 1, 2 , Hui Yang 3 , Weiyi Guo 4 , Lingmei Guan 1 , Hai Wu 1 , Libo Du 1
Chemical Research in Toxicology ( IF 4.1 ) Pub Date : 2020-03-03 , DOI: 10.1021/acs.chemrestox.9b00368 Xiaojie Zhang 1 , Xueling Guo 1 , Xiaoxuan Kang 1, 2 , Hui Yang 3 , Weiyi Guo 4 , Lingmei Guan 1 , Hai Wu 1 , Libo Du 1
Affiliation
|
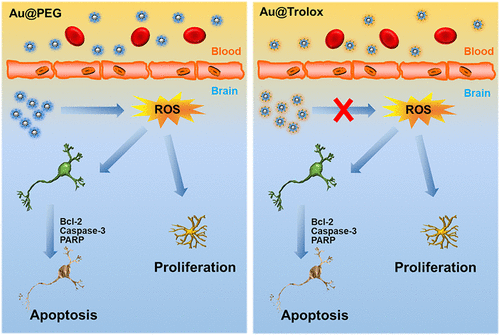
Because of their biocompatibility and biosafety, pegylated Au NPs (Au@PEG), as a nanodrug-carrier, have been widely applied in different biomedical applications, including imaging and drug delivery systems. Under such conditions, the biosafety of Au@PEG has attracted tremendous attention. However, only a small number of studies focused on the neurotoxicity of Au@PEG used as drug delivery carriers not to mention reducing the neurotoxicity of Au@PEG. To address this issue, the adverse effects of Au@PEG on human neuroblastoma SHSY5Y cells were first investigated. The results showed that 4.5 nm Au@PEG significantly induced cell apoptosis through upregulating reactive oxygen species (ROS) production and disordering the mitochondrial membrane potential. To further evaluate whether the neurotoxicity of Au@PEG could be improved through conjugating antioxidants on the surface of Au@PEG, Trolox (a vitamin E analogue)-functionalized Au@PEG (Au@Trolox) was synthesized. The results showed that the neurotoxicity of Au@PEG on SHSY5Y cells could be significantly improved by Au@Trolox. Next, mice were subjected to administration of 4.5 nm Au@PEG and Au@Trolox for 3 months. An increase of oxidative stress and a decrease in the activity of key antioxidant enzymes including glutathione peroxidase (GSH-Px), superoxide dismutase (SOD), and catalase (CAT) were observed after long-term injection of Au@PEG. More importantly, both the apoptosis of neurons and the activation of astrocytes were observed in the hippocampus of mice injected with Au@PEG. In contrast, the adverse effects of Au@PEG could be improved when injected with Au@Trolox. In short, the present study provided new insights into the toxicity evaluation of nanoparticles and would help to better understand and prevent the neurotoxicity of nanomaterials used in pharmaceutics.
中文翻译:

具有抗氧化剂的聚乙二醇化金纳米颗粒的表面功能化抑制了纳米颗粒诱导的氧化应激和神经毒性。
由于它们的生物相容性和生物安全性,作为纳米药物载体的聚乙二醇化金纳米颗粒(Au @ PEG)已广泛应用于包括成像和药物输送系统在内的各种生物医学应用中。在这样的条件下,Au @ PEG的生物安全性引起了极大的关注。然而,只有少数研究集中在用作药物递送载体的Au @ PEG的神经毒性上,更不用说降低Au @ PEG的神经毒性了。为了解决这个问题,首先研究了Au @ PEG对人神经母细胞瘤SHSY5Y细胞的不利影响。结果表明,4.5 nm Au @ PEG通过上调活性氧(ROS)的产生和破坏线粒体膜电位而诱导细胞凋亡。为了进一步评估是否可以通过在Au @ PEG表面缀合抗氧化剂来改善Au @ PEG的神经毒性,合成了Trolox(一种维生素E类似物)官能化的Au @ PEG(Au @ Trolox)。结果表明Au @ Trolox可以明显改善Au @ PEG对SHSY5Y细胞的神经毒性。接下来,给小鼠施用4.5nm的Au @ PEG和Au @ Trolox 3个月。长期注射Au @ PEG后,观察到氧化应激的增加和关键抗氧化剂酶的活性降低,包括谷胱甘肽过氧化物酶(GSH-Px),超氧化物歧化酶(SOD)和过氧化氢酶(CAT)。更重要的是,在注射Au @ PEG的小鼠的海马中观察到了神经元的凋亡和星形胶质细胞的激活。相反,注射Au @ Trolox可以改善Au @ PEG的副作用。简而言之,本研究为纳米颗粒的毒性评估提供了新的见识,并将有助于更好地理解和预防用于药物的纳米材料的神经毒性。
更新日期:2020-03-03
中文翻译:

具有抗氧化剂的聚乙二醇化金纳米颗粒的表面功能化抑制了纳米颗粒诱导的氧化应激和神经毒性。
由于它们的生物相容性和生物安全性,作为纳米药物载体的聚乙二醇化金纳米颗粒(Au @ PEG)已广泛应用于包括成像和药物输送系统在内的各种生物医学应用中。在这样的条件下,Au @ PEG的生物安全性引起了极大的关注。然而,只有少数研究集中在用作药物递送载体的Au @ PEG的神经毒性上,更不用说降低Au @ PEG的神经毒性了。为了解决这个问题,首先研究了Au @ PEG对人神经母细胞瘤SHSY5Y细胞的不利影响。结果表明,4.5 nm Au @ PEG通过上调活性氧(ROS)的产生和破坏线粒体膜电位而诱导细胞凋亡。为了进一步评估是否可以通过在Au @ PEG表面缀合抗氧化剂来改善Au @ PEG的神经毒性,合成了Trolox(一种维生素E类似物)官能化的Au @ PEG(Au @ Trolox)。结果表明Au @ Trolox可以明显改善Au @ PEG对SHSY5Y细胞的神经毒性。接下来,给小鼠施用4.5nm的Au @ PEG和Au @ Trolox 3个月。长期注射Au @ PEG后,观察到氧化应激的增加和关键抗氧化剂酶的活性降低,包括谷胱甘肽过氧化物酶(GSH-Px),超氧化物歧化酶(SOD)和过氧化氢酶(CAT)。更重要的是,在注射Au @ PEG的小鼠的海马中观察到了神经元的凋亡和星形胶质细胞的激活。相反,注射Au @ Trolox可以改善Au @ PEG的副作用。简而言之,本研究为纳米颗粒的毒性评估提供了新的见识,并将有助于更好地理解和预防用于药物的纳米材料的神经毒性。


























 京公网安备 11010802027423号
京公网安备 11010802027423号