当前位置:
X-MOL 学术
›
ACS Med. Chem. Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
HOPPI-NMR: Hot-Peptide-Based Screening Assay for Inhibitors of Protein-Protein Interactions by NMR.
ACS Medicinal Chemistry Letters ( IF 4.2 ) Pub Date : 2020-02-20 , DOI: 10.1021/acsmedchemlett.9b00620 Diego Brancaccio 1 , Salvatore Di Maro 2 , Linda Cerofolini 3 , Stefano Giuntini 3 , Marco Fragai 3 , Claudio Luchinat 3 , Stefano Tomassi 1 , Antonio Limatola 4 , Pasquale Russomanno 1 , Francesco Merlino 1 , Ettore Novellino 1 , Alfonso Carotenuto 1
ACS Medicinal Chemistry Letters ( IF 4.2 ) Pub Date : 2020-02-20 , DOI: 10.1021/acsmedchemlett.9b00620 Diego Brancaccio 1 , Salvatore Di Maro 2 , Linda Cerofolini 3 , Stefano Giuntini 3 , Marco Fragai 3 , Claudio Luchinat 3 , Stefano Tomassi 1 , Antonio Limatola 4 , Pasquale Russomanno 1 , Francesco Merlino 1 , Ettore Novellino 1 , Alfonso Carotenuto 1
Affiliation
|
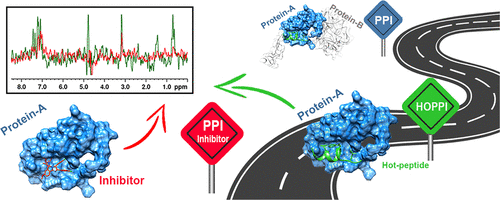
Protein-protein interactions (PPIs) contribute to the onset and/or progression of several diseases, especially cancer, and this discovery has paved the way for considering disruption of the PPIs as an attractive anti-tumor strategy. In this regard, simple and efficient biophysical methods for detecting the interaction of the inhibitors with the protein counterpart are still in high demand. Herein, we describe a convenient NMR method for the screening of putative PPI inhibitors based on the use of "hot peptides" (HOPPI-NMR). As a case study, HOPPI-NMR was successful applied to the well-known p53/MDM2 system. Our outcomes highlight the main advantages of the method, including the use of a small amount of unlabeled proteins, the minimization of the risk of protein aggregation, and the ability to identify weak binders. The last leaves open the possibility for application of HOPPI-NMR in tandem with fragment-based drug discovery as a valid strategy for the identification of novel chemotypes acting as PPI inhibitors.
中文翻译:

HOPPI-NMR:通过NMR对蛋白质-蛋白质相互作用的抑制剂进行基于热肽的筛选分析。
蛋白质-蛋白质相互作用(PPI)助长了几种疾病(尤其是癌症)的发作和/或进展,这一发现为将PPI破坏视为有吸引力的抗肿瘤策略铺平了道路。在这方面,仍然需要用于检测抑制剂与蛋白质对应物相互作用的简单有效的生物物理方法。在此,我们描述了一种基于“热肽”(HOPPI-NMR)筛选推定的PPI抑制剂的简便NMR方法。作为一个案例研究,HOPPI-NMR成功应用于著名的p53 / MDM2系统。我们的成果突出了该方法的主要优势,包括使用少量未标记的蛋白质,将蛋白质聚集的风险降至最低以及鉴定弱结合剂的能力。
更新日期:2020-02-20
中文翻译:

HOPPI-NMR:通过NMR对蛋白质-蛋白质相互作用的抑制剂进行基于热肽的筛选分析。
蛋白质-蛋白质相互作用(PPI)助长了几种疾病(尤其是癌症)的发作和/或进展,这一发现为将PPI破坏视为有吸引力的抗肿瘤策略铺平了道路。在这方面,仍然需要用于检测抑制剂与蛋白质对应物相互作用的简单有效的生物物理方法。在此,我们描述了一种基于“热肽”(HOPPI-NMR)筛选推定的PPI抑制剂的简便NMR方法。作为一个案例研究,HOPPI-NMR成功应用于著名的p53 / MDM2系统。我们的成果突出了该方法的主要优势,包括使用少量未标记的蛋白质,将蛋白质聚集的风险降至最低以及鉴定弱结合剂的能力。


























 京公网安备 11010802027423号
京公网安备 11010802027423号