Journal of Investigative Dermatology ( IF 6.5 ) Pub Date : 2020-03-02 , DOI: 10.1016/j.jid.2020.01.032 Uffe Høgh Olesen 1 , Gael Clergeaud 2 , Kristoffer Kjærgaard Hendel 1 , Kelvin Yeung 1 , Catharina Margrethe Lerche 1 , Thomas Lars Andresen 2 , Merete Haedersdal 1
|
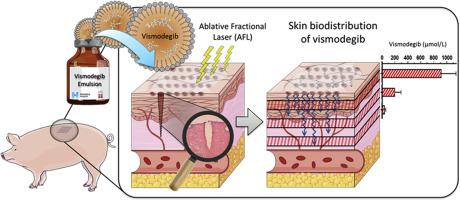
Oral administration of vismodegib for basal cell carcinoma treatment is limited by significant class-specific systemic side effects. We investigated the approach of combining ablative fractional laser–assisted drug delivery with an extended-release microemulsion formulation of vismodegib to provide efficient cutaneous delivery in vivo. The developed formulation consisted of an oil-in-water microemulsion stabilized by Tween-80. Pig skin was exposed to ablative fractional laser followed by topical application of vismodegib microemulsion for 4 hours. At 4 hours, 2 days, 5 days, and 9 days, we evaluated vismodegib biodistribution in superficial, mid, and deep dermis and plasma (n = 189 measurements) and assessed local skin reactions. Sustained topical delivery of vismodegib was detected in all depths of ablative fractional laser–exposed skin over the course of the study, with peak concentrations found at 5 days and 9 days. The highest vismodegib concentrations reached 1,409.7 μmol/liter in superficial dermis and 62.3 μmol/liter in deep dermis, exceeding steady-state plasma concentrations previously reported for oral administration of vismodegib (5.5–56.0 μmol/liter). Ablative fractional laser increased vismodegib uptake up to 16.6-fold compared with intact skin. Only mild local skin responses to vismodegib were observed, and no vismodegib was detected in plasma. We report sustained topical delivery of vismodegib in vivo at high concentrations with favorable skin tolerability, suggesting a future safer vismodegib treatment.
中文翻译:

烧蚀性分批激光和微乳剂配方可增强和维持维他命单抗的皮肤递送。
vismodegib用于基底细胞癌治疗的口服给药受到明显的类特异性全身性副作用的限制。我们研究了将烧蚀性激光辅助药物递送与vismodegib的缓释微乳剂配方结合以在体内提供有效皮肤递送的方法。开发的配方由通过Tween-80稳定的水包油型微乳液组成。猪皮肤暴露于烧蚀性分级激光,然后局部应用维斯莫德微乳4小时。在4小时,2天,5天和9天时,我们评估了vismodegib在浅表层,中层和深层真皮和血浆中的生物分布(n = 189个测量值),并评估了局部皮肤反应。在整个研究过程中,在剥蚀性激光照射的所有深度的皮肤中均检测到维斯莫吉布的持续局部递送,并在5天和9天达到峰值浓度。最高的维斯莫德布浓度在浅层真皮中达到1,409.7μmol/升,在深层真皮中达到62.3μmol/升,超过了以前口服维斯莫德布的稳态血药浓度(5.5-56.0μmol/升)。与完整的皮肤相比,烧蚀性分数激光使vismodegib摄取增加了16.6倍。仅观察到对vismodegib的轻微局部皮肤反应,并且在血浆中未检测到vismodegib。我们报告了高浓度维索莫昔布在体内持续局部递送,具有良好的皮肤耐受性,表明未来的维莫希德布治疗更安全。在5天和9天时达到峰值浓度。最高的维斯莫德布浓度在浅层真皮中达到1,409.7μmol/升,在深层真皮中达到62.3μmol/升,超过了以前口服维斯莫德布的稳态血药浓度(5.5–56.0μmol/升)。与完整的皮肤相比,烧蚀性分数激光使vismodegib摄取增加了16.6倍。仅观察到对vismodegib的轻微局部皮肤反应,在血浆中未检测到vismodegib。我们报告高浓度维索莫昔单抗在体内持续局部递送,具有良好的皮肤耐受性,表明未来的维莫昔单抗治疗更安全。在5天和9天时达到峰值浓度。最高的维斯莫德布浓度在浅层真皮中达到1,409.7μmol/升,在深层真皮中达到62.3μmol/升,超过了以前口服维斯莫德布的稳态血药浓度(5.5–56.0μmol/升)。与完整的皮肤相比,烧蚀性分数激光使vismodegib摄取增加了16.6倍。仅观察到对vismodegib的轻微局部皮肤反应,在血浆中未检测到vismodegib。我们报告高浓度维索莫昔单抗在体内持续局部递送,具有良好的皮肤耐受性,表明未来的维莫昔单抗治疗更安全。超过以前报道的维莫昔单抗口服稳态血药浓度(5.5-56.0μmol/升)。与完整的皮肤相比,烧蚀性分数激光使vismodegib摄取增加了16.6倍。仅观察到对vismodegib的轻微局部皮肤反应,在血浆中未检测到vismodegib。我们报告了高浓度维索莫昔布在体内持续局部递送,具有良好的皮肤耐受性,表明未来的维莫希德布治疗更安全。超过以前报道的维莫昔单抗口服稳态血药浓度(5.5-56.0μmol/升)。与完整的皮肤相比,烧蚀性分数激光使vismodegib摄取增加了16.6倍。仅观察到对vismodegib的轻微局部皮肤反应,在血浆中未检测到vismodegib。我们报告高浓度维索莫昔单抗在体内持续局部递送,具有良好的皮肤耐受性,表明未来的维莫昔单抗治疗更安全。


























 京公网安备 11010802027423号
京公网安备 11010802027423号