当前位置:
X-MOL 学术
›
J. Chem. Educ.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Changes of CO2 Concentration and Heat Illustrate Why the Flame Is Extinguished in the Candle-and-Cylinder Experiment
Journal of Chemical Education ( IF 3 ) Pub Date : 2020-03-02 , DOI: 10.1021/acs.jchemed.9b00833 Ronghui Que 1 , Sha Sha 1 , Liqun Shen 1 , Yanlin Xiong 1
Journal of Chemical Education ( IF 3 ) Pub Date : 2020-03-02 , DOI: 10.1021/acs.jchemed.9b00833 Ronghui Que 1 , Sha Sha 1 , Liqun Shen 1 , Yanlin Xiong 1
Affiliation
|
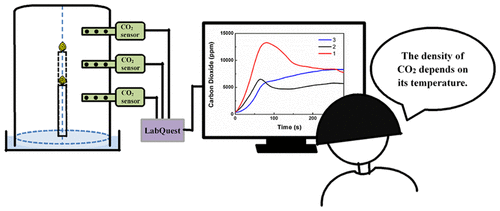
The extinguishment of the candle flame in the well-known candle-and-cylinder experiment has been erroneously viewed as caused by the complete consumption of oxygen, for many reasons. To address this misconception scientifically, a series of experiments are carried out to illustrate the phenomenon from a new point of view. Limewater [Ca(OH)2] placed at different heights in a container was used to qualitatively describe the distribution of CO2 in an enclosed cylinder with one or two burning candles. Temperature sensors, CO2 sensors, and oxygen (O2) sensors were employed to visually and quantitatively compare the content of CO2 at different heights within the container. The resulting temperature curves show that the heat from combustion rose first and then descended soon afterward. Chemical and sensor detection of CO2 in the highest two quadrants of the container indicated that the hot CO2 gas rose initially and accumulated there in the highest part of the container. Then, the hot CO2 gas descended gradually and created a CO2 atmosphere surrounding the flame. As a result, the flame was extinguished by the buildup of CO2 and depletion of oxygen in the gas in the vicinity of the flame. The change of oxygen concentration showed that there was a mixing process between the remaining air and the hot CO2. These results indicate that the extinguishment of the flame does not mean that the oxygen in the container was completely consumed. Knowing this helps students understand that the density of CO2 depends on its temperature.
中文翻译:

CO 2浓度和热量的变化说明了烛形和筒形实验中火焰被熄灭的原因
由于许多原因,人们已经错误地认为在众所周知的烛缸实验中熄灭烛火是由于氧气的完全消耗引起的。为了科学地解决这一误解,我们进行了一系列实验,从新的角度说明了这一现象。使用放置在容器中不同高度的柠檬水[Ca(OH)2 ]定性地描述在带有一两个燃烧蜡烛的密闭圆筒中CO 2的分布。使用温度传感器,CO 2传感器和氧气(O 2)传感器来视觉和定量比较CO 2的含量在容器内的不同高度。所得的温度曲线表明,燃烧产生的热量先上升,然后很快下降。化学和传感器检测CO的2在容器的最高两个象限表明热CO 2气体最初上升和容器中的最高的部分积累在那里。然后,热的CO 2气体逐渐下降并在火焰周围形成CO 2气氛。结果,由于火焰附近的气体中CO 2的积累和氧气的耗尽,火焰被熄灭。氧气浓度的变化表明,剩余空气与热CO 2之间存在混合过程。这些结果表明,火焰的熄灭并不意味着容器中的氧气已被完全消耗掉。知道这一点有助于学生理解CO 2的密度取决于其温度。
更新日期:2020-04-24
中文翻译:

CO 2浓度和热量的变化说明了烛形和筒形实验中火焰被熄灭的原因
由于许多原因,人们已经错误地认为在众所周知的烛缸实验中熄灭烛火是由于氧气的完全消耗引起的。为了科学地解决这一误解,我们进行了一系列实验,从新的角度说明了这一现象。使用放置在容器中不同高度的柠檬水[Ca(OH)2 ]定性地描述在带有一两个燃烧蜡烛的密闭圆筒中CO 2的分布。使用温度传感器,CO 2传感器和氧气(O 2)传感器来视觉和定量比较CO 2的含量在容器内的不同高度。所得的温度曲线表明,燃烧产生的热量先上升,然后很快下降。化学和传感器检测CO的2在容器的最高两个象限表明热CO 2气体最初上升和容器中的最高的部分积累在那里。然后,热的CO 2气体逐渐下降并在火焰周围形成CO 2气氛。结果,由于火焰附近的气体中CO 2的积累和氧气的耗尽,火焰被熄灭。氧气浓度的变化表明,剩余空气与热CO 2之间存在混合过程。这些结果表明,火焰的熄灭并不意味着容器中的氧气已被完全消耗掉。知道这一点有助于学生理解CO 2的密度取决于其温度。



























 京公网安备 11010802027423号
京公网安备 11010802027423号