当前位置:
X-MOL 学术
›
Adv. Synth. Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
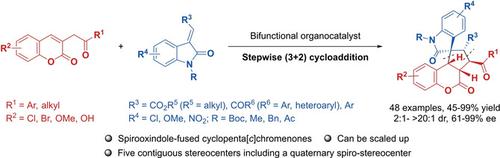
Enantioselective Construction of Spirooxindole‐Fused Cyclopenta[c]chromen‐4‐ones Bearing Five Contiguous Stereocenters via a Stepwise (3+2) Cycloaddition
Advanced Synthesis & Catalysis ( IF 5.4 ) Pub Date : 2020-03-23 , DOI: 10.1002/adsc.201901655 Sandip Sambhaji Vagh, Praneeth Karanam, Cheng‐Chieh Liao, Ting‐Han Lin, Yan‐Cheng Liou, Athukuri Edukondalu, Yi‐Ru Chen, Wenwei Lin
Advanced Synthesis & Catalysis ( IF 5.4 ) Pub Date : 2020-03-23 , DOI: 10.1002/adsc.201901655 Sandip Sambhaji Vagh, Praneeth Karanam, Cheng‐Chieh Liao, Ting‐Han Lin, Yan‐Cheng Liou, Athukuri Edukondalu, Yi‐Ru Chen, Wenwei Lin
|
The bifunctional quinine‐catalyzed stepwise (3+2) cycloaddition for the enantioselective construction of spirooxindole‐fused cyclopenta[c]chromen‐4‐ones is developed. The reactions of 3‐homoacylcoumarins and alkylidene oxindole electrophiles generate aforementioned spirooxindole‐chromenone adducts bearing five contiguous stereocenters, of which one is the spiro all‐carbon quaternary stereocenter in high yields (up to 99%) with excellent stereoselectivities (up to >20:1 dr and 99% ee). This methodology was investigated for three different alkylidene oxindole electrophiles and could also be practically demonstrated on a gram scale. Mechanistic investigations revealed that the (3+2) cycloaddition for the enantioselective synthesis of spirooxindole‐fused cyclopenta[c]chromen‐4‐ones is proceeding via a stepwise reaction pathway.
中文翻译:

通过逐步(3 + 2)环加成反应,构筑具有五个连续立体中心的螺氧杂吲哚稠合的环戊[c] chromen-4-ones的对映选择性构建
开发了双功能奎宁催化的逐步(3 + 2)环加成反应,用于螺氧基吲哚稠合的环戊多[ c ] chromen -4-ones的对映选择性构建。3-高酰基香豆素与亚烷基氧吲哚亲电子的反应生成上述带有5个连续立体中心的螺杂吲哚-色酮酮加合物,其中一个是高产率(高达99%)的螺全碳四元立体中心,具有出色的立体选择性(高达> 20: 1博士和99%ee)。已针对三种不同的亚烷基ind吲哚亲电子试剂研究了该方法,并且也可以在克级上进行实际证明。机理研究表明,(3 + 2)环加成反应可合成螺氧杂吲哚稠合的环戊五烯[ c]] chromen-4-ones通过逐步反应途径进行。
更新日期:2020-04-21
中文翻译:

通过逐步(3 + 2)环加成反应,构筑具有五个连续立体中心的螺氧杂吲哚稠合的环戊[c] chromen-4-ones的对映选择性构建
开发了双功能奎宁催化的逐步(3 + 2)环加成反应,用于螺氧基吲哚稠合的环戊多[ c ] chromen -4-ones的对映选择性构建。3-高酰基香豆素与亚烷基氧吲哚亲电子的反应生成上述带有5个连续立体中心的螺杂吲哚-色酮酮加合物,其中一个是高产率(高达99%)的螺全碳四元立体中心,具有出色的立体选择性(高达> 20: 1博士和99%ee)。已针对三种不同的亚烷基ind吲哚亲电子试剂研究了该方法,并且也可以在克级上进行实际证明。机理研究表明,(3 + 2)环加成反应可合成螺氧杂吲哚稠合的环戊五烯[ c]] chromen-4-ones通过逐步反应途径进行。



























 京公网安备 11010802027423号
京公网安备 11010802027423号