Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Plasmonic thermochromism based on a reversible redox reaction of Ag+/Ag on Au nanorods
Nanoscale ( IF 6.7 ) Pub Date : 2020/02/29 , DOI: 10.1039/d0nr00117a Hao Xie 1, 2, 3, 4, 5 , Pengyu Xu 4, 5, 6, 7, 8 , Fei Zhao 1, 2, 3, 4, 5 , Haifei Zhu 1, 2, 3, 4, 5 , Kaiyu Wang 1, 2, 3, 4, 5 , Weixiang Ye 1, 2, 3, 4, 5 , Weihai Ni 1, 2, 3, 4, 5
Nanoscale ( IF 6.7 ) Pub Date : 2020/02/29 , DOI: 10.1039/d0nr00117a Hao Xie 1, 2, 3, 4, 5 , Pengyu Xu 4, 5, 6, 7, 8 , Fei Zhao 1, 2, 3, 4, 5 , Haifei Zhu 1, 2, 3, 4, 5 , Kaiyu Wang 1, 2, 3, 4, 5 , Weixiang Ye 1, 2, 3, 4, 5 , Weihai Ni 1, 2, 3, 4, 5
Affiliation
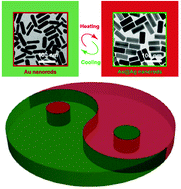
|
Reversible redox reaction-based thermochromism using plasmonic nanocrystals is challenging due to the requirements set based on the complexity of the reaction system where the oxidizing and reducing agents must not interfere with each other, and both should possess temperature sensitivity. Herein, we demonstrate plasmonic thermochromism based on a reversible redox reaction of Ag+/Ag on Au nanorods (AuNRs) by incorporating temperature-sensitive reducing and oxidizing agents into the same system. The competition between reduction and oxidation is solely dependent on temperature. When the temperature is above (below) the transition temperature, the reduction of Ag+ (oxidation of Ag) dominates on the surface of AuNRs, and the thermochromic nanostructure solution appears green (red). An experimental study on the mechanism reveals that HOCl produced at low concentrations by H2O2 is the source of the observed temperature dependence of the Ag oxidation. Rationally tuning the transition temperature in a range from 27 to 40 °C can be realized by changing the concentration of some key chemical compounds in the solution. The thermochromic solution can be standalone-functional within multiple cycles of heating and cooling and long-term storage without any additional reagents. Our study provides new insight into plasmonic thermochromism and may pave the way for fabricating smart thermochromic materials.
中文翻译:

基于Ag + / Ag在Au纳米棒上的可逆氧化还原反应的等离子热变色
由于基于反应体系的复杂性而设定的要求(氧化剂和还原剂不得相互干扰,并且两者都应具有温度敏感性),因此使用等离激元纳米晶体的基于可逆氧化还原反应的热致变色极具挑战性。在这里,我们通过将对温度敏感的还原剂和氧化剂引入同一系统,证明了基于Ag + / Ag在Au纳米棒(AuNRs)上可逆氧化还原反应的等离子体热致变色。还原和氧化之间的竞争仅取决于温度。当温度高于(低于)转变温度时,Ag +的还原(Ag的氧化)在AuNRs的表面占主导地位,热致变色纳米结构溶液显示为绿色(红色)。对该机理的实验研究表明,H 2 O 2在低浓度下生成的HOCl是观察到的Ag氧化温度依赖性的来源。通过改变溶液中某些关键化合物的浓度,可以在27至40°C的范围内合理地调节转变温度。该热致变色溶液可以在加热和冷却以及长期存储的多个循环中独立运行,而无需任何其他试剂。我们的研究为等离子热致变色提供了新的见识,并可能为制造智能热致变色材料铺平道路。
更新日期:2020-04-03
中文翻译:

基于Ag + / Ag在Au纳米棒上的可逆氧化还原反应的等离子热变色
由于基于反应体系的复杂性而设定的要求(氧化剂和还原剂不得相互干扰,并且两者都应具有温度敏感性),因此使用等离激元纳米晶体的基于可逆氧化还原反应的热致变色极具挑战性。在这里,我们通过将对温度敏感的还原剂和氧化剂引入同一系统,证明了基于Ag + / Ag在Au纳米棒(AuNRs)上可逆氧化还原反应的等离子体热致变色。还原和氧化之间的竞争仅取决于温度。当温度高于(低于)转变温度时,Ag +的还原(Ag的氧化)在AuNRs的表面占主导地位,热致变色纳米结构溶液显示为绿色(红色)。对该机理的实验研究表明,H 2 O 2在低浓度下生成的HOCl是观察到的Ag氧化温度依赖性的来源。通过改变溶液中某些关键化合物的浓度,可以在27至40°C的范围内合理地调节转变温度。该热致变色溶液可以在加热和冷却以及长期存储的多个循环中独立运行,而无需任何其他试剂。我们的研究为等离子热致变色提供了新的见识,并可能为制造智能热致变色材料铺平道路。



























 京公网安备 11010802027423号
京公网安备 11010802027423号