Molecular Metabolism ( IF 8.1 ) Pub Date : 2020-02-29 , DOI: 10.1016/j.molmet.2020.02.012 Cuiying Xiao 1 , Naili Liu 2 , Haley Province 1 , Ramón A Piñol 1 , Oksana Gavrilova 2 , Marc L Reitman 1
|
Objective
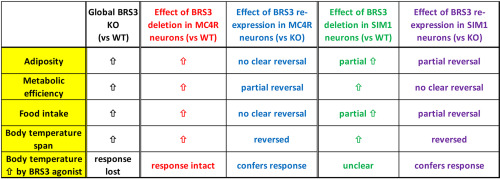
Bombesin-like receptor 3 (BRS3) is an orphan receptor and Brs3 knockout mice develop obesity with increased food intake and reduced resting metabolic rate and body temperature. The neuronal populations contributing to these effects were examined.
Methods
We studied energy metabolism in mice with Cre-mediated recombination causing 1) loss of BRS3 selectively in SIM1- or MC4R-expressing neurons or 2) selective re-expression of BRS3 from a null background in these neurons.
Results
The deletion of BRS3 in MC4R neurons increased body weight/adiposity, metabolic efficiency, and food intake, and reduced insulin sensitivity. BRS3 re-expression in these neurons caused partial or no reversal of these traits. However, these observations were confounded by an obesity phenotype caused by the Mc4r-Cre allele, independent of its recombinase activity. The deletion of BRS3 in SIM1 neurons increased body weight/adiposity and food intake, but not to the levels of the global null. The re-expression of BRS3 in SIM1 neurons reduced body weight/adiposity and food intake, but not to wild type levels. The deletion of BRS3 in either MC4R- or SIM1-expressing neurons affected body temperature, with re-expression in either population reversing the null phenotype. MK-5046, a BRS3 agonist, increases light phase body temperature in wild type, but not Brs3 null, mice and BRS3 re-expression in either population restored response to MK-5046.
Conclusions
BRS3 in both MC4R- and SIM1-expressing neurons contributes to regulation of body weight/adiposity, insulin sensitivity, food intake, and body temperature.
中文翻译:

表达MC4R和SIM1的神经元中的BRS3调节小鼠体内的能量稳态。
目的
Bombesin样受体3(BRS3)是一种孤儿受体,Brs3基因敲除小鼠会因食物摄入增加,静息代谢率和体温降低而发生肥胖。检查了促成这些作用的神经元群体。
方法
我们研究了Cre介导的重组小鼠中的能量代谢,这种代谢导致1)在表达SIM1或MC4R的神经元中选择性丢失BRS3,或2)从这些神经元中的空背景选择性地重新表达BRS3。
结果
MC4R神经元中BRS3的缺失增加了体重/肥胖,代谢效率和食物摄入,并降低了胰岛素敏感性。这些神经元中BRS3的重新表达导致这些性状的部分或完全没有逆转。但是,这些观察结果与由Mc4r-Cre引起的肥胖表型混淆等位基因,与其重组酶活性无关。SIM1神经元中BRS3的缺失增加了体重/肥胖和食物摄入量,但没有达到总体无效水平。在SIM1神经元中BRS3的重新表达减少了体重/肥胖和食物摄入,但没有达到野生型水平。表达MC4R或SIM1的神经元中BRS3的缺失会影响体温,而任一群体中的重新表达都会逆转无效表型。BRS3激动剂MK-5046在野生型中会增加光相体温,但不会使Brs3 null消失,这两个种群中的小鼠和BRS3重新表达都恢复了对MK-5046的反应。
结论
表达MC4R和SIM1的神经元中的BRS3有助于调节体重/肥胖,胰岛素敏感性,食物摄入和体温。


























 京公网安备 11010802027423号
京公网安备 11010802027423号