European Journal of Medicinal Chemistry ( IF 6.7 ) Pub Date : 2020-02-28 , DOI: 10.1016/j.ejmech.2020.112191 Alexandra Knox , Christina Kalchschmid , Daniela Schuster , Francesca Gaggia , Claudia Manzl , Daniel Baecker , Ronald Gust
|
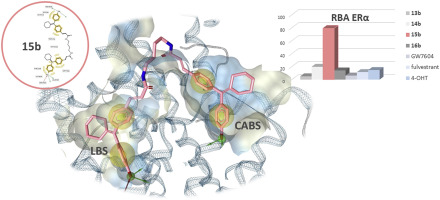
Up to 80% of mammary carcinoma initially exhibit estrogen-dependent growth, which can be treated by aromatase inhibitors or SERMs/SERDs. To increase the options after failure of the hormonal therapy with these drugs, the search for alternatives with a different mode of action to prevent estrogen action is of high relevance. Therefore, this study focused on the inhibition of coactivator recruitment at the estrogen receptor (ER) by targeted attachment of bivalent compounds at the coactivator binding site besides the primary binding at the ligand binding domain. Eight homodimeric 4-[1-(4-hydroxyphenyl)-2-phenyl-1-butenyl]cinnamic acid (GW7604)- or cyclofenilacrylic acid-based ER ligands with diaminoalkane linkers (C2–C5) were synthesized and their effects on the ER subtypes were assessed in vitro. All compounds possessed full antagonistic potency at ERα/β as determined in a transactivation assay. Furthermore, they exerted medium downregulatory effects dependent on the spacer length and did not stimulate the ER expression as observed for 4-hydroxytamoxifen. The cyclofenil-derived dimer with C4 spacer (15b) showed the highest binding affinity to ERα (RBA = 79.2%) and downregulated the ER content in MCF-7 cells with an efficiency of 38% at 1 μM.
中文翻译:

二价三芳基烯烃和环苯腈衍生的双重雌激素受体拮抗剂和下调剂的开发
最初有多达80%的乳腺癌表现出雌激素依赖性生长,可以通过芳香化酶抑制剂或SERM / SERD进行治疗。为了在激素治疗失败后增加这些药物的选择,寻找具有不同作用方式以预防雌激素作用的替代物具有高度相关性。因此,该研究集中于通过在配体结合结构域上的主要结合之外,将二价化合物靶向附着在共激活剂结合位点上,抑制在雌激素受体(ER)上共激活剂募集。合成了具有二氨基烷烃连接基(C2-C5)的八个同二聚4- [1-(4-羟基苯基)-2-苯基-1-丁烯基]肉桂酸(GW7604)-或基于环苯腈丙烯酸的ER配体对亚型进行了体外评估。如反式激活法所测定,所有化合物对ERα/β具有完全的拮抗作用。此外,它们根据间隔物长度发挥中等的下调作用,并且不刺激4-羟基他莫昔芬的ER表达。带有C4间隔基的环苯醌衍生的二聚体(15b)对ERα的结合亲和力最高(RBA = 79.2%),并在1μM时下调了MCF-7细胞中ER的含量,效率为38%。



























 京公网安备 11010802027423号
京公网安备 11010802027423号