当前位置:
X-MOL 学术
›
J. Comput. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Aspartate: An interesting model for analyzing dipole-ion and ion pair interactions through its oppositely charged amine and acid groups
Journal of Computational Chemistry ( IF 3 ) Pub Date : 2020-02-28 , DOI: 10.1002/jcc.26184 Belén Hernández 1, 2 , Fernando Pflüger 2 , Mahmoud Ghomi 1, 2
Journal of Computational Chemistry ( IF 3 ) Pub Date : 2020-02-28 , DOI: 10.1002/jcc.26184 Belén Hernández 1, 2 , Fernando Pflüger 2 , Mahmoud Ghomi 1, 2
Affiliation
|
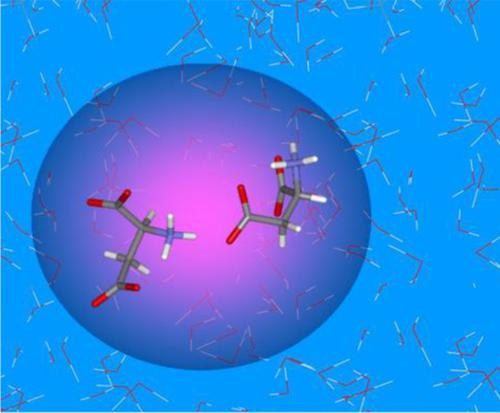
Anionic species of aspartic acid, Asp−, having a zwitterionic backbone and a deprotonated side chain, appears to be a good example for analyzing dipole‐ion and ion pair interactions. Density functional theory calculations were herein performed to investigate the low energy conformers of Asp− embedded in a dielectric continuum modeling an aqueous environment, through a scan of the potential energy as a function of the side chain (χ1, χ2) torsion angles. The most energetically favorable conformers having g+g− and g−g+ side chain orientations are found to be stabilized by charge‐enhanced intramolecular H‐bonding involving the positively charged ( NH3+ ) and the two negatively charged (COO−) groups. These conformers were further used to analyze Asp− + nW clusters (W: water, n = 1 or 3), and Asp−/Asp− pair formation. COO− groups were found to be the most attractive sites for hosting a water molecule (binding energy: −6.0 ± 1.5 kcal/mol), compared to NH3+ groups (binding energy: −4.7 ± 1.1 kcal/mol). Energy separation between g+g− and g−g+ conformers increases upon explicit hydration. Asp−/Asp− ion pairs, stabilized by the interaction between the NH3+ group of a partner and the COO− group of the other, shows a quite constant binding energy (−8.1 ± 0.2 kcal/mol), whatever the pair type, and the relative orientation of the two interacting partners. This study suggests a first step to achieve a more realistic image of intermolecular interactions in aqueous environment, especially upon increasing concentration. It can also be considered as a preliminary attempt to assess the interactions of the Lys+…Asp−/Glu− ion pairs stabilizing intra‐ and interchain interactions in proteins.
中文翻译:

天冬氨酸:通过其带相反电荷的胺和酸基团分析偶极离子和离子对相互作用的有趣模型
具有两性离子主链和去质子化侧链的天冬氨酸阴离子物质 Asp− 似乎是分析偶极离子和离子对相互作用的一个很好的例子。本文进行了密度泛函理论计算,以通过扫描作为侧链 (χ1, χ2) 扭转角函数的势能来研究嵌入介电连续体中的 Asp- 的低能量构象异构体,模拟水性环境。发现具有 g+g- 和 g-g+ 侧链取向的能量最有利的构象异构体通过电荷增强的分子内氢键稳定,包括带正电的 (NH3+) 和两个带负电的 (COO-) 基团。这些构象异构体进一步用于分析 Asp- + nW 簇(W:水,n = 1 或 3)和 Asp-/Asp- 对的形成。与 NH3+ 基团(结合能:-4.7 ± 1.1 kcal/mol)相比,发现 COO− 基团是最吸引水分子的位点(结合能:-6.0 ± 1.5 kcal/mol)。g+g− 和 g−g+ 构象异构体之间的能量分离在显式水合时增加。Asp-/Asp- 离子对,通过一个伙伴的 NH3+ 基团和另一个 COO- 基团之间的相互作用稳定,显示出相当恒定的结合能 (-8.1 ± 0.2 kcal/mol),无论对类型如何,并且两个互动伙伴的相对取向。这项研究表明,第一步是在水性环境中获得更真实的分子间相互作用图像,尤其是在浓度增加时。
更新日期:2020-02-28
中文翻译:

天冬氨酸:通过其带相反电荷的胺和酸基团分析偶极离子和离子对相互作用的有趣模型
具有两性离子主链和去质子化侧链的天冬氨酸阴离子物质 Asp− 似乎是分析偶极离子和离子对相互作用的一个很好的例子。本文进行了密度泛函理论计算,以通过扫描作为侧链 (χ1, χ2) 扭转角函数的势能来研究嵌入介电连续体中的 Asp- 的低能量构象异构体,模拟水性环境。发现具有 g+g- 和 g-g+ 侧链取向的能量最有利的构象异构体通过电荷增强的分子内氢键稳定,包括带正电的 (NH3+) 和两个带负电的 (COO-) 基团。这些构象异构体进一步用于分析 Asp- + nW 簇(W:水,n = 1 或 3)和 Asp-/Asp- 对的形成。与 NH3+ 基团(结合能:-4.7 ± 1.1 kcal/mol)相比,发现 COO− 基团是最吸引水分子的位点(结合能:-6.0 ± 1.5 kcal/mol)。g+g− 和 g−g+ 构象异构体之间的能量分离在显式水合时增加。Asp-/Asp- 离子对,通过一个伙伴的 NH3+ 基团和另一个 COO- 基团之间的相互作用稳定,显示出相当恒定的结合能 (-8.1 ± 0.2 kcal/mol),无论对类型如何,并且两个互动伙伴的相对取向。这项研究表明,第一步是在水性环境中获得更真实的分子间相互作用图像,尤其是在浓度增加时。



























 京公网安备 11010802027423号
京公网安备 11010802027423号